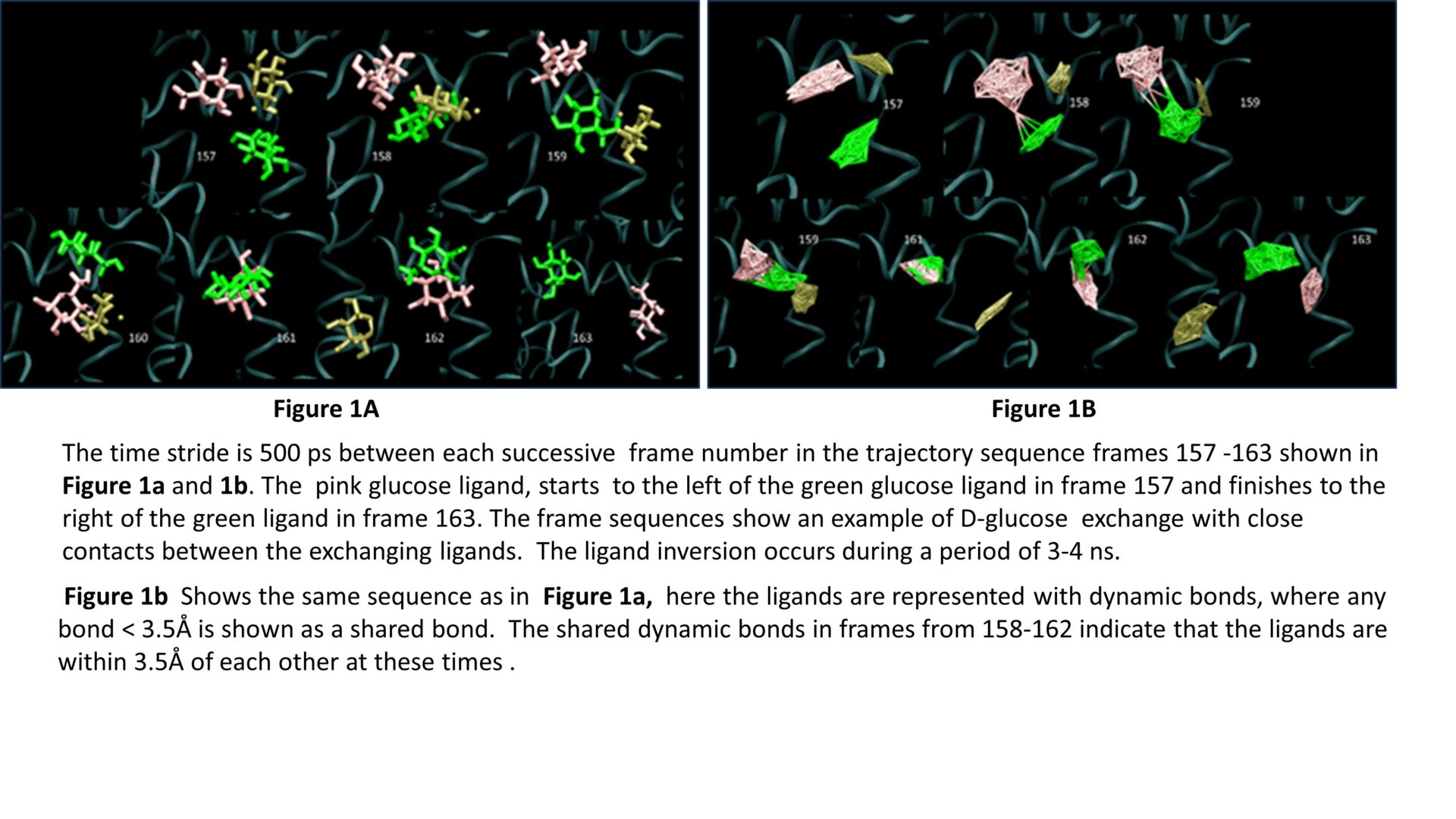
Atomistic molecular dynamics simulations demonstrate that when multiple β-D-glucose molecules are present within the GLUT1 transporter, simultaneous position exchanges frequently occur between adjacent ligands. These exchanges take place in the internal cavities and at both external and internal solution interfaces of the protein. They involve rotation of adjacent ligand positions along the central pore axis of the transporter with variable duration in the nanoscale (4 – 100 ns). Exchanges occurring at the extracellular protein interfaces involve fast displacements (2 – 10 ns) of D-glucose H-bonded to the protein interface by other D-glucose molecules present in solution. These examples of simultaneous D-glucose exchanges demonstrate that accelerated exchange is consistent with a multisite model for D-glucose transport within GLUTs where multiple D-glucose molecules move independently and stochastically within the transporter’s tunnels, cavities, and the central pore.
Higher frequency of D-glucose exchange is observed in the membrane gel state, corresponding with D-glucose transport in human erythrocytes at low temperatures. The presence of multiple D-glucose molecules both within the transporter and in bathing solutions increases D-glucose penetration depths from the solutions into transporter intramembranous zones, particularly in the gel state.
That exchange frequency between adjacent ligands depends on the local D-glucose density within the transport pathway explains why accelerated exchange occurs more frequently in conditions where bottlenecks at the openings of the transport pathway are prolonged, at low temperatures (1), thereby augmenting ligand aggregation in the adjacent upstream regions.