Insulin resistance and type 2 diabetes have been associated with interleukin (IL)-6 gene polymorphism (Fernandez-Real et al. 2000), higher plasma concentrations of IL-6 (Pickup et al. 1997) and IL-6 release from adipose tissue (Kern et al. 2001). However, the evidence linking IL-6 expression in skeletal muscle to insulin resistance is poor. The aim of this study, therefore, was to examine the effect of insulin resistance and hyperinsulinaemia on skeletal muscle IL-6 expression. The study was approved by The University of Melbourne, Animal Ethics Committee.
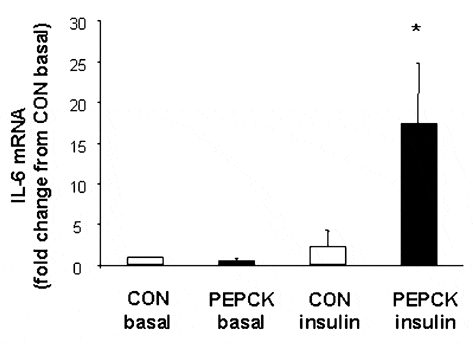
Transgenic rats were rendered insulin resistant in skeletal muscle due to an over-expression of the gluconeogenic regulatory enzyme phospho-enolpyruvate carboxykinase (PEPCK) in the kidney and liver. White gastrocnemius muscle samples were obtained from animals aged 10-12 weeks. Samples were obtained under hyperinsulinaemic, euglycaemic clamp (4 mU kg-1 min-1 insulin, plasma glucose concentration 4-6 mmol l-1) and basal conditions in both PEPCK (n = 5) and wild-type control (CON) (n = 5) rats and measured for IL-6 mRNA. Data were analysed using a two-way (group X treatment) ANOVA. No differences in IL-6 mRNA expression were observed when comparing CON with PEPCK under basal conditions. In addition, insulin stimulation in CON did not change IL-6 gene expression compared with basal levels. In contrast, there was a ~17-fold increase (P < 0.05) in IL-6 gene expression in insulin-stimulated PEPCK compared with CON basal levels (see Fig. 1).
These data demonstrate that in highly glycolytic, white muscle, insulin stimulation of non-insulin resistant skeletal muscle tissue alone does not alter IL-6 gene expression and that peripheral insulin resistance does not alter IL-6 gene expression under basal conditions. However, insulin stimulation of peripherally insulin resistant muscle increases IL-6 gene expression above that of the same tissue under basal conditions, and above that of non-insulin resistant rodent white gastrocnemius muscle under insulin stimulation. The function of the increase in IL-6 in insulin-resistant muscle subjected to insulin stimulation is unknown. However, the recent observations that (1) muscle contraction rapidly increases both IL-6 gene expression and nuclear transcriptional activity; (2) both are augmented when muscle glycogen availability is reduced and (3) the increased expression of IL-6 is associated with increased glucose uptake during exercise (Keller et al. 2001; Steensberg et al. 2001), suggest that the up-regulation of IL-6 in insulin-resistant, insulin stimulated skeletal muscle is a consequence rather than a cause of insulin resistance and that the function is to enhance glucose uptake when the demand for glucose disposal is increased.