Since synaptic vesicles in the brain and in the neuromuscular junction have an electrolucent interior when viewed under the electron microscope, it is generally supposed they are filled with an aqueous solution containing neurotransmitters and cotransmitters at a high concentration. However, other secretory granules from mast cells and chromaffin cells are filled with a matrix which adsorbs bioamines by electrostatic forces (çlvarez de Toledo et al. 1993; Marszalek et al. 1997). The existence of such a matrix has not been considered in synaptic vesicles. Of interest here is the fact that ATP is stored in all synaptic vesicles independently of neurotransmitter type, making this molecule a marker for synaptic vesicular content. We chose the electric organ of Torpedo for the present study because the synaptic vesicles fraction is easily isolated. Cholinergic synaptic vesicles of Torpedo electric organ store acetylcholine (ACh) and ATP at a concentration near the molar range. These molecules are co-secreted during synaptic transmission, and the release of both can be monitored via specific luminescence assays. Here we study the release of ACh or ATP from permeabilized synaptic vesicles in conditions that mimic the interior of the synaptic vesicle and in the absence of small ions.
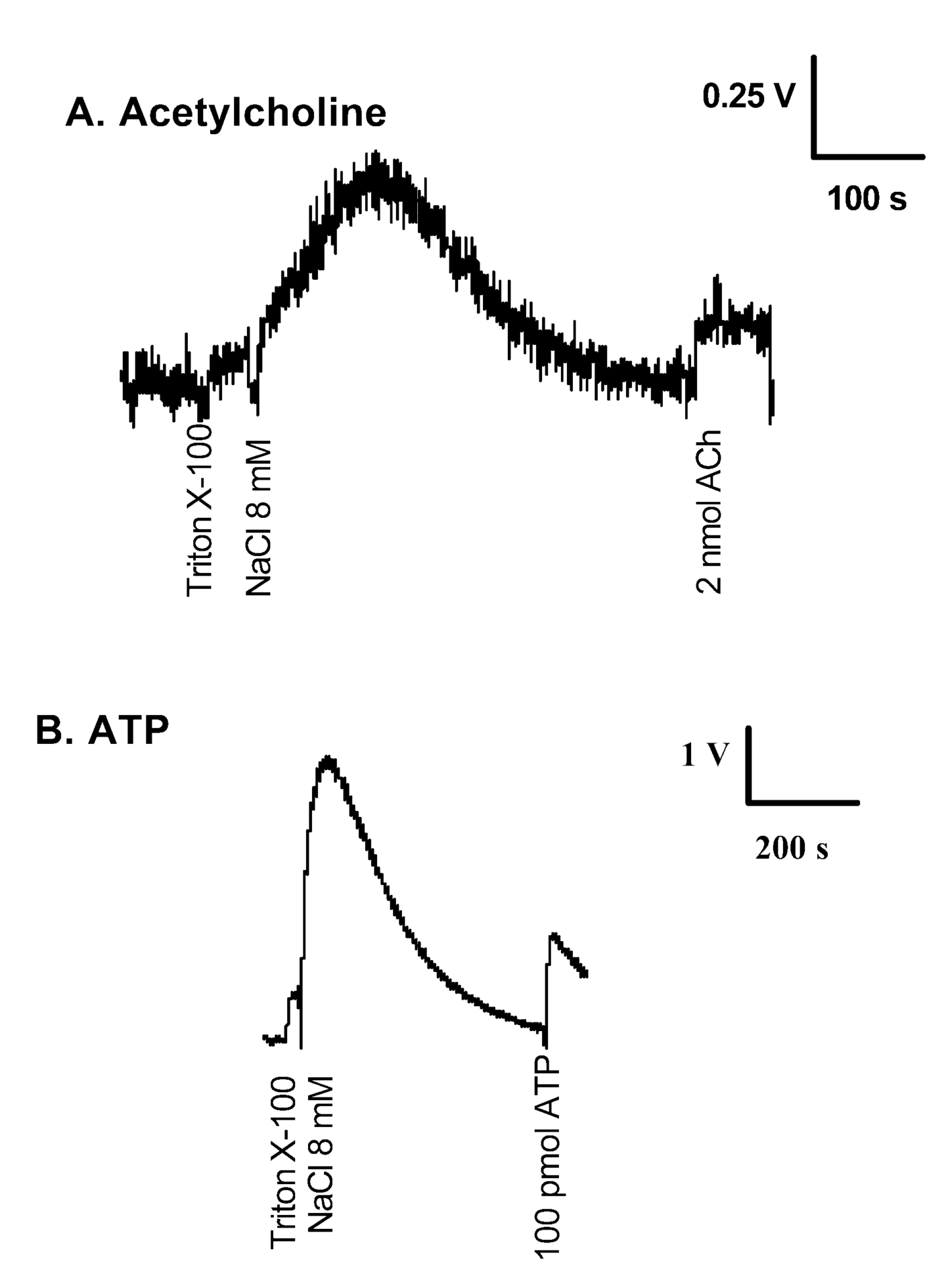
Electric organs were removed from Torpedo sp. specimens anaesthetized with tricaine (3 g l-1 sea water, MS-222, Sigma,). Synaptic vesicle suspensions were extensively dialysed in isotonic media free of small ions (Cl–, Na+, K+, Ca2+). When dialysed synaptic vesicles were permeabilized with Triton X-100 (Fig. 1A), a small release of ACh was detected (6.9 ± 1.5 nmol ACh (mg protein)-1, n = 3; 0.45 ± 0.06 nmol ATP (mg protein)-1, n = 4). By comparison, when these permeabilized vesicles were further tested with an additional pulse of NaCl (8 mM), a 10-fold increase of ACh was detected (62 ± 12 nmol ACh (mg protein)-1, n = 3 and 6.95 ± 0.56 nmol ATP (mg protein)-1, n = 10).
This result suggests that neurotransmitters are not in a free solution in the vesicles, but are electrostatically adherent to some support. The calculated EC50 for NaCl was 2.7 mM. Other salts containing other ions were also tested, and similar results were obtained. Interestingly, the EC50 for CaCl2 was 0.3 mM. We ruled out the possibility that the detergent may have interfered with the ionic environment or with the luminescent reaction by bursting synaptic vesicles in ultrapure water. The amount of ATP released (Fig. 1B) was 0.54 ± 0.07 nmol ATP (mg protein)-1 (n = 3), which corresponded to the free ATP inside the synaptic vesicles. An additional bolus of NaCl (8 mM) evoked a second peak of ATP release (9.9 ± 0.93 nmol ATP (mg protein)-1, n = 3), which emptied the synaptic vesicles. The ratio of free ATP/total ATP in synaptic vesicles burst by ultrapure water was similar (5.45 %, n = 3) to that measured in detergent-permeabilized vesicles. We examined whether the synaptic vesicles lysed by ultrapure water could be refilled after a run of release of ACh and ATP. The water-lysed synaptic vesicle fraction was emptied of ACh and ATP by increasing the NaCl concentration and was refilled for a second time with ACh or ATP by dialysis combination. When maintained in ultrapure water, the refilled matrix kept up a high content of ATP, at least for a few days. However, the addition of a low concentration of NaCl induced the release of ATP. As in the case of intact vesicles, the release of ATP was also salt- concentration dependent. The pretreatment of lysed vesicles with EGTA (10 mM) completely prevents the process of ATP refilling. With respect to ACh refilling, a lysed and emptied synaptic vesicle fraction was refilled with ACh, and the addition of NaCl induced the release of ACh.
This study was funded by MCyT from the Spanish Government, CIRIT from the Generalitat de Catalunya and Fundació La Marató de TV3.