J.A.F. holds a Research Fellowship at Gonville & Caius College, Cambridge. J.A.U-S. thanks Astra Zeneca and acknowledges additional support from the James Baird Fund. C.L.-H.H. thanks the Medical Research Council, the Wellcome Trust and the British Heart Foundation.
University of Heidelberg (2006) Proc Physiol Soc 4, C5
Oral Communications: James A. Fraser1, Juliet A. Usher-Smith1, Christopher L. Huang1
1. Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, United Kingdom.
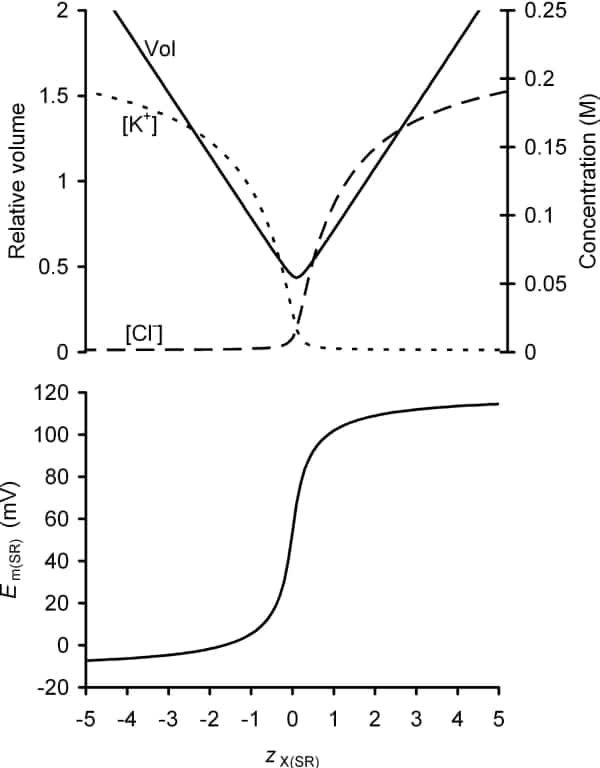
A number of previous reports suggested that skeletal muscle sarcoplasmic reticular (SR) membranes are permeable to ions such as K+ and Cl- (1), but do not contain mechanisms for their active transport. This is compatible with their passive distribution in a Donnan equilibrium across the SR membrane in quiescent muscle. A theoretical study using a multi-compartment Gibbs-Donnan analysis and charge-difference modelling (2) was used to explore the determinants of SR luminal [K+] and [Cl-], SR membrane potential and SR volume in a system of this kind. It demonstrated that the principal parameters that influenced these key SR variables were the net charge carried by normally membrane-impermeant ions within the SR (such as Ca2+ and calsequestrin) and the extracellular membrane-permeant ion concentrations and osmolarity. Strikingly, SR concentrations of passively distributed ions were not influenced by sarcoplasmic ion concentrations or by the surface membrane potential. These findings facilitate an understanding of the charge balancing that must occur following changes in SR Ca2+ content. In particular, they demonstrate (Fig. 1) that if the SR membrane potential in resting muscle is to be close to zero, as observed experimentally (3), then the net charge carried by membrane-impermeant ions (X) within the SR must be similar to that of the membrane-impermeant ions within the sarcoplasm and therefore negative. Figure 1 further provides a possible explanation for the observed SR volume increase following significant Ca2+ release (4): such loss of Ca2+, which is normally membrane-impermeant, would tend to make the mean charge per osmole of membrane-impermeant ions within the SR (zX(SR)) more negative. Conversely, Ca2+ overload of the SR might increase zX(SR) sufficiently to render the SR membrane potential significantly positive, contributing to the observed increases in Ca2+ release (5). This novel application of charge difference modelling thus provides a simple physiological basis for a number of key, yet hitherto unexplained, observations concerning SR volume and membrane potential.
View other abstracts by:
Figure 1. The predicted influence of the mean charge per osmole of intra-SR membrane-impermeant ions (zX(SR)) upon SR volume SR membrane potential (Em(SR)) and intra-SR ion concentrations calculated using Gibbs-Donnan relationships. Note that the SR membrane is normally impermeable to Ca2+ at rest and total SR Ca2+ content (buffered + free) is high. Thus zX(SR) is likely to be profoundly influenced by the intra-SR Ca2+ content.
Where applicable, experiments conform with Society ethical requirements.