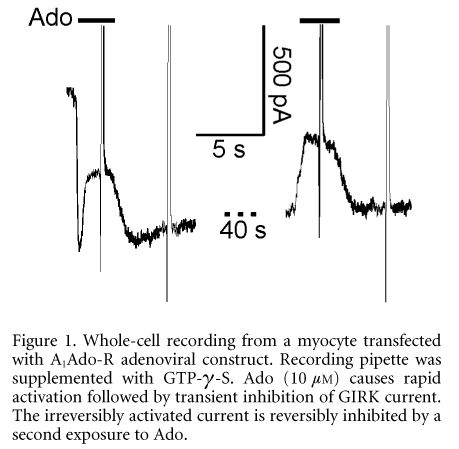
GIRK channels in atrial myocytes are activated by various G-protein-coupled receptors including muscarinic M2 (M2AChR) and purinergic A1 (A1AdoR). In adult rat atrial myocytes the amplitude of GIRK current evoked by adenosine is limited by the density of A1AdoR to ~30 % of the current in the presence of ACh at a saturating concentration. Transfection of atrial myocytes with conventional vectors encoding for A1AdoR increases sensitivity to adenosine (Wellner-Kienitz et al. 2000). In the present study we confirmed these results by means of a more efficient adenovirus-based method of gene transfer (He et al. 1998). Adult rats were anaesthetized and killed by cervical dislocation. The heart was removed for isolation of myocytes. In a fraction of A1AdoR transfected myocytes, displaying a particularly high fluorescence-intensity of the reporter protein (GFP), activation of GIRK current by Ado (10 mM) was followed by a rapid decay (t1/2 ~ 300 ms). Upon washout of Ado a rebound of current was observed that was proportional to the degree of the initial decay. In those cells Ado caused an inhibition of M2AChR-induced current in the continuous presence of ACh. In GTP-λ-S-loaded myocytes stably and irreversibly activated GIRK current was reversibly inhibited by Ado (Fig. 1).
Upon activation of endogenous M2AChR by a highly saturating concentration of ACh (1 mM), a similar inhibitory component can be detected. These results suggest a novel mechanism of GIRK current inhibition by G-protein-coupled receptors, which requires fast activation of a large number of receptors, and which seems to be independent of GTP cycling of a heterotrimeric G-protein.
This work was supported by Deutsche Forschungsgemeinschaft.
All procedures accord with current National guidelines.