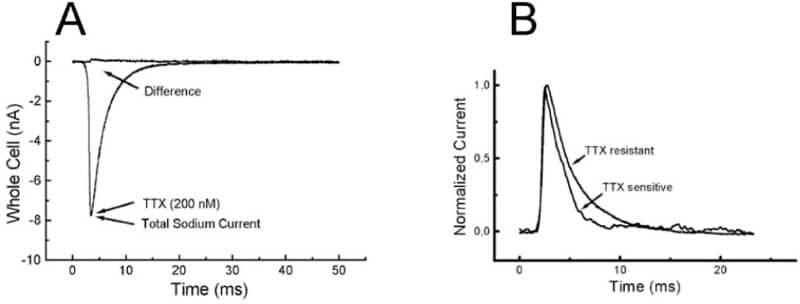
In the heart, excitation-contraction coupling begins with the activation of sodium channels underlying the rapid rise of action potential and its propagation. Classical cardiac sodium channels are tetrodotoxin (TTX)-insensitive but recent cytoimmunochemical labelling (Maier et al., 2002) suggest the presence of neuronal-type sodium channels in the t-tubules whereas TTX-resistant channels are preferentially localised at intercalated disks. However, the interpretation of immunochemical data as regards the precise localisation of sparse membrane proteins is not straightforward. We thus set out to obtain more direct functional evidence using ‘Scanning Ion Conductance Microscopy’ (SICM) combined with ‘smart patch-clamp’ as applied for calcium channels in heart cells (Shevchuk et al., 2001). SICM images were recorded from both control and detubulated ventricular cardiomyocytes (Brette et al. 2002) isolated from Spague-Dawley rats. This permitted patch t-tubules openings in addition to the classical patching at random on the cell surface. With grayanotoxin (100 μM) in the pipette allowing steady-state activation, single-channel events of 11 pS were observed with a higher rate of success when patching t-tubules openings. We then compared the TTX-sensitivity of whole-cell sodium currents of control and detubulated cells. Bath application of 200 nM TTX reduced the sodium current peak of control cells (Fig. 1A) by 18±5 % (SEM, n = 12) whereas the effect was insignificant in detubulated cells. This was matched by a parallel decrease of membrane capacitance of ∼30% for the detubulated cells. The peak of the remaining current of control cells (after TTX addition) occurred slightly earlier than the total sodium current and fast inactivation decay was accelerated (1.3 vs. 3 ms, Fig. 1B), as expected for neuronal, TTX-sensitive sodium channels. This study provides independent functional evidence for neuronal-type sodium channels in heart cells where they play the likely role of rapidly and synchronously couple t-tubules and cell surface depolarisations.
King's College London (2005) J Physiol 565P, C84
Communications: Localisation of neuronal sodium channels in rat ventricular cardiomyocytes
Duclohier, Herve Pierre Marie; Yang, Lin Q; Gorelik, Julia ; Harding, Sian E; Korchev, Yuri E;
1. NHLI Division, Imperial College, LONDON, United Kingdom. 2. MRC - Clinical Sciences Centre, Imperial College, LONDON, United Kingdom. 3. Institut de Physiologie et Biologie Cellulaire, UMR 6187 CNRS - Universite de Poitiers , POITIERS, France.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.