Background: White adipose tissue (WAT) stores excess energy and acts as an endocrine organ releasing hormone-like factors known as adipokines that regulate whole-body energy balance. Brown adipose tissue (BAT) regulates energy expenditure through futile cycling of the electron transport chain (ETC) by the action of uncoupling protein 1 (UCP1) to generate heat. BAT’s heat producing capacity may be a novel approach to treating obesity. Adult humans possess metabolically active BAT, but obesity reduces BAT’s thermogenic activity and mass causing BAT to resemble WAT in a process termed “whitening”. Leucine-rich-α2-glycoprotein 1 (Lrg1) is an adipose-associated secretory protein and potential adipokine, with plasma levels positively correlated to body mass index in humans. However, the role of LRG1 in the regulation of BAT and sWAT energy metabolism and function has not yet been identified.
Hypothesis: LRG1 may function to inhibit BAT thermogenesis and energy expenditure and drive whitening of BAT. We proposed that inhibiting Lrg1 may drive BAT thermogenesis with anti-obesity and anti-diabetic effects and thereby identify LRG1 as a therapeutic target.
Methods: Live animal experiments were performed in accordance with Animals (Scientific Procedures) Act 1986. Male Lrg1 global knockout (KO) mice or wild type (WT) controls were fed standard chow (STD) or high fat diet (HFD) for 10 weeks (n=10). At 18 weeks of age, systemic and BAT metabolic characteristics were determined by whole body indirect calorimetry, high-resolution tissue respirometry, quantitative PCR, histology and immunoblots. Within WAT. fatty acid β-oxidation was assessed using functional assays. Statistical significance was assessed using one-way ANOVA with Dunnett’s multiple comparison test or two-way ANOVA with Sidak’s multiple comparisons test.
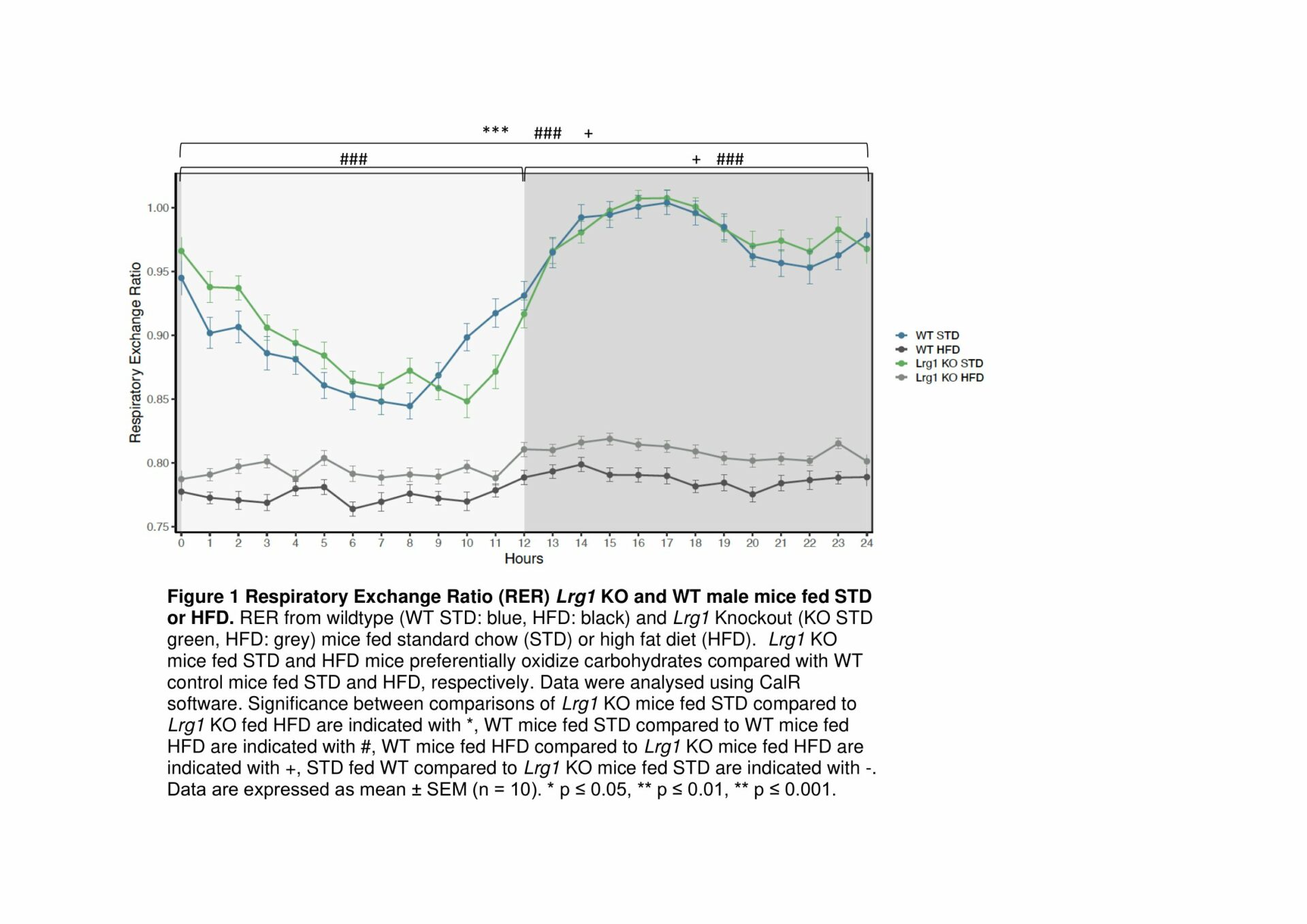
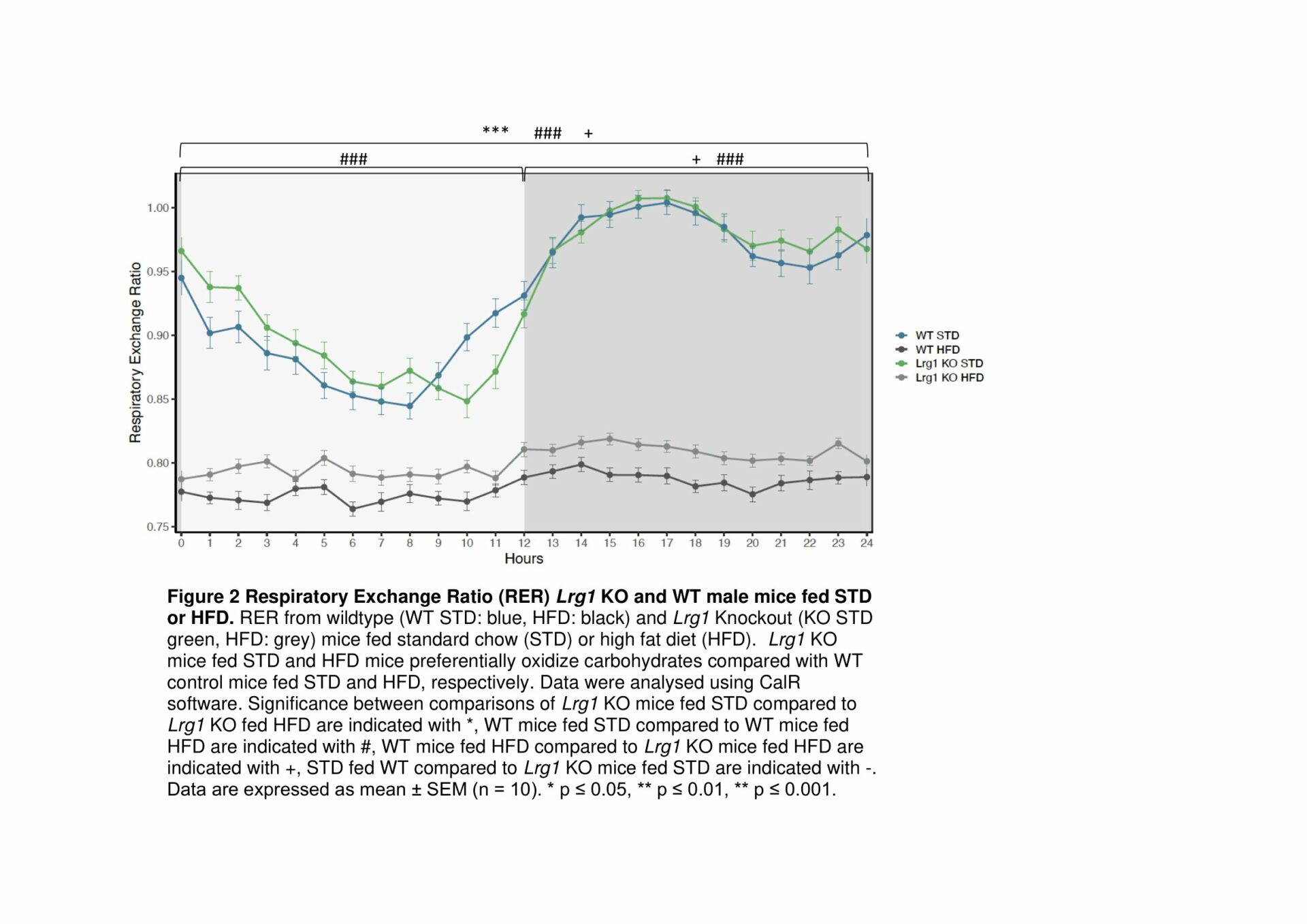
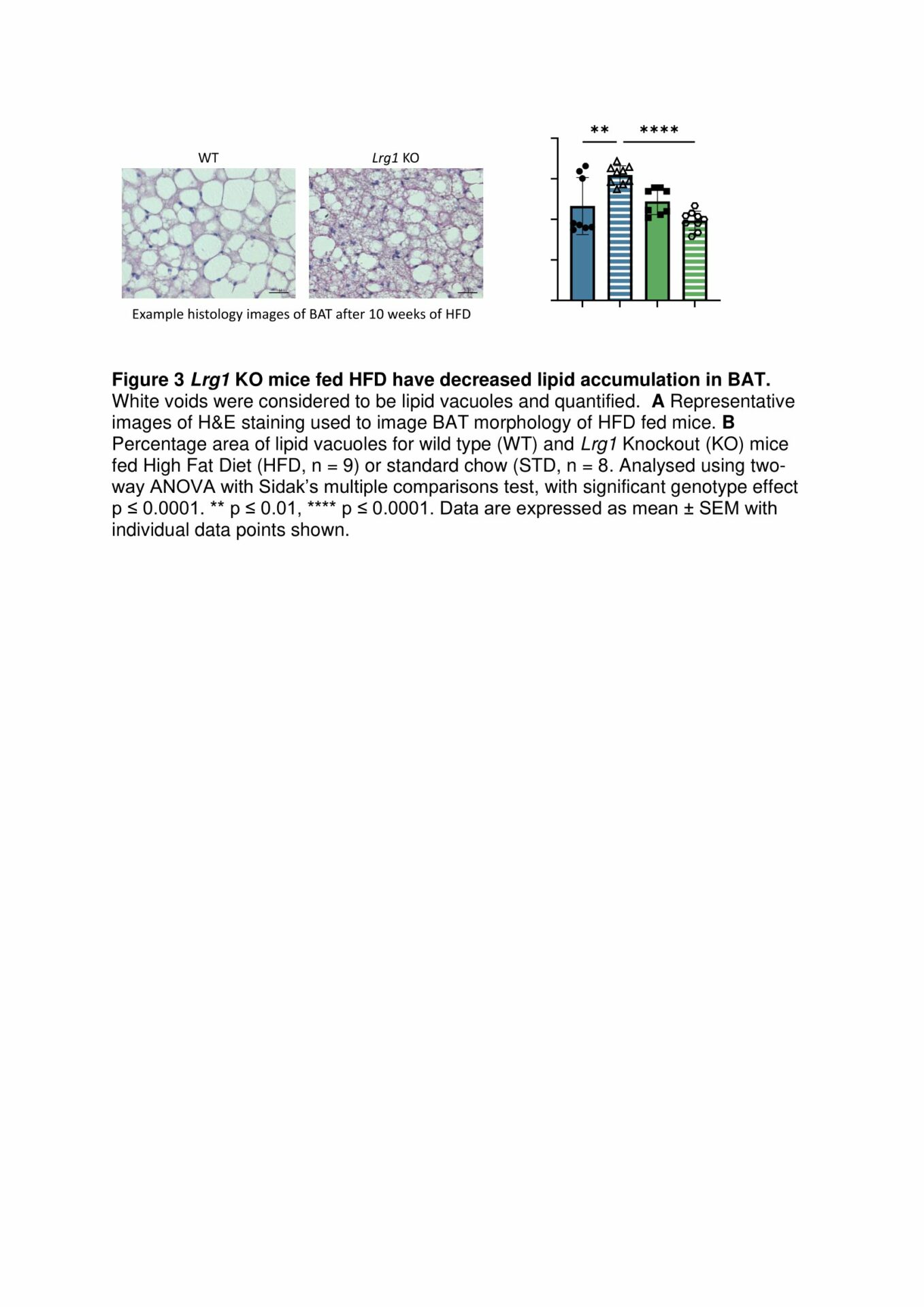
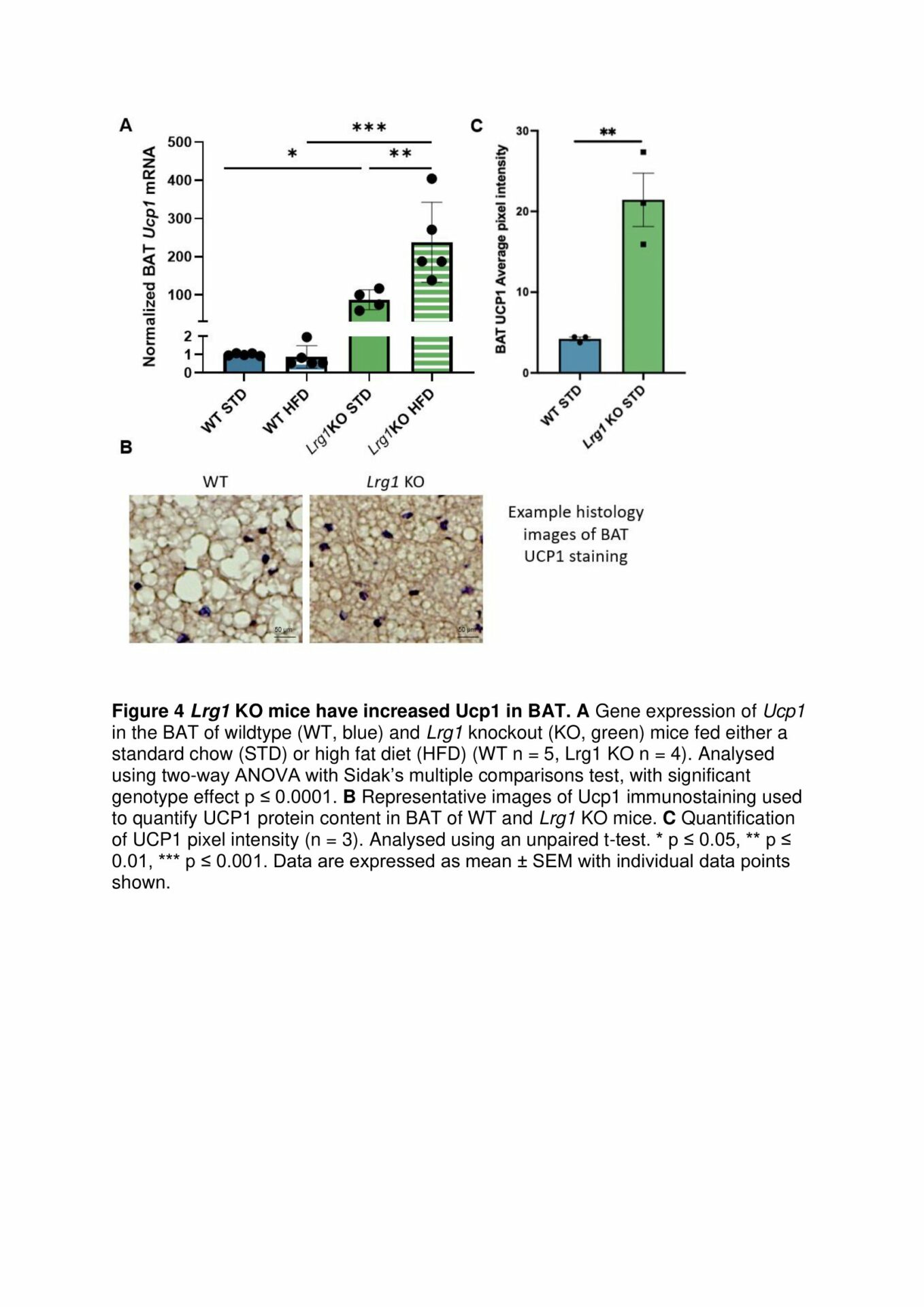
Results: BAT from HFD-fed WT mice exhibit a decrease in mitochondrial respiration through complex 1 of the ETC, demonstrating that loss of LRG1 protects HFD-induced dysfunction in BAT (Fig. 1). Functional loss of Lrg1 induces a systemic shift towards preferential lipid oxidation in Lrg1 KO mice with both HFD and STD-fed mice having a lower respiratory exchange ratio (RER) than WT controls (Fig. 2). Lrg1 KO mice fed HFD have greater BAT wet weight WT mice. H&E histology confirmed that the heavier BAT in Lrg1 KO mice was not due to whitening of BAT (Fig. 3). Lrg1 null mice exhibit a molecular phenotype indicative of thermogenic futile cycling, which was observed through increased Ucp1 gene and protein expression in the Lrg1 KO mice (Fig. 4).
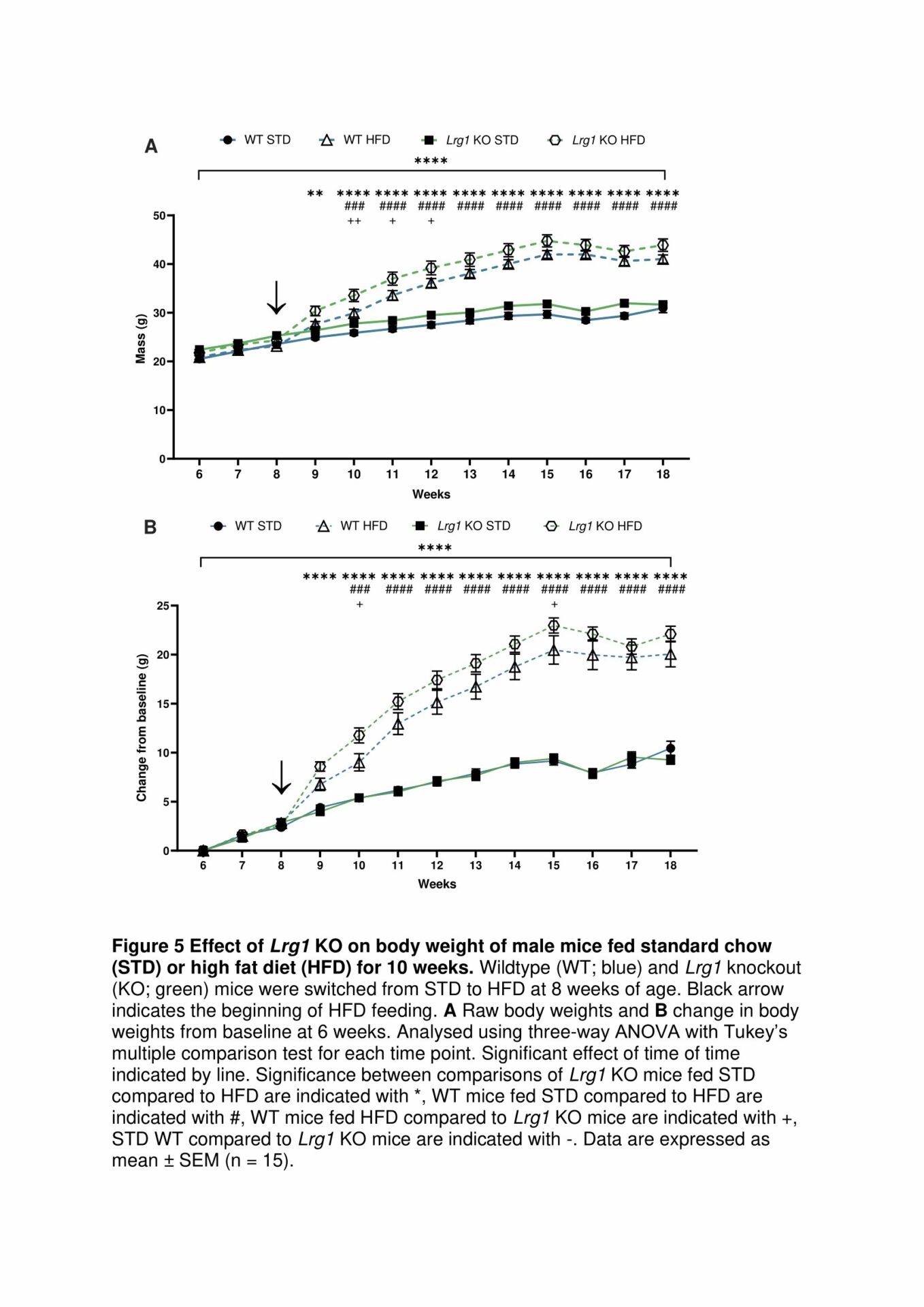
However, Lrg1 KO does not protect against weight gain in a mouse model of diet-induced obesity (Fig. 5). Lrg1 null mice have reduced thermogenic gene expression in white adipose tissue. Lrg1 null mice have impaired fatty acid β-oxidation in subcutaneous adipose tissue, demonstrated through a reduction in cellular lipid uptake, reduced Cpt1a gene expression, and reduced CPT1a activity.
Conclusion: Loss of Lrg1 increased BAT UCP1 expression, increased mitochondrial respiration and decreased BAT whitening in models of obesity, indicating that loss of LRG1 increases BAT thermogenesis and protects against obesity-induced dysfunction. However, increased BAT thermogenesis did not lead to reduced weight gain due to decreased fatty acid β-oxidation within sWAT of Lrg1 null mice.