Continuous assessment and monitoring of symptom fluctuations and disease progression in Parkinson’s Disease (PD) can improve treatment and provide robust outcome measures for clinical trials, making them faster and more conclusive. Despite impressive progression over the past decade, this remains a challenge, and the gold standard for clinical trials is still clinical assessment based on the Unified Parkinson’s Disease Rating Scale (UPDRS), which is subjective and conducted at scattered intervals, failing to adequately capture daily symptoms fluctuations in symptoms experienced by patients.
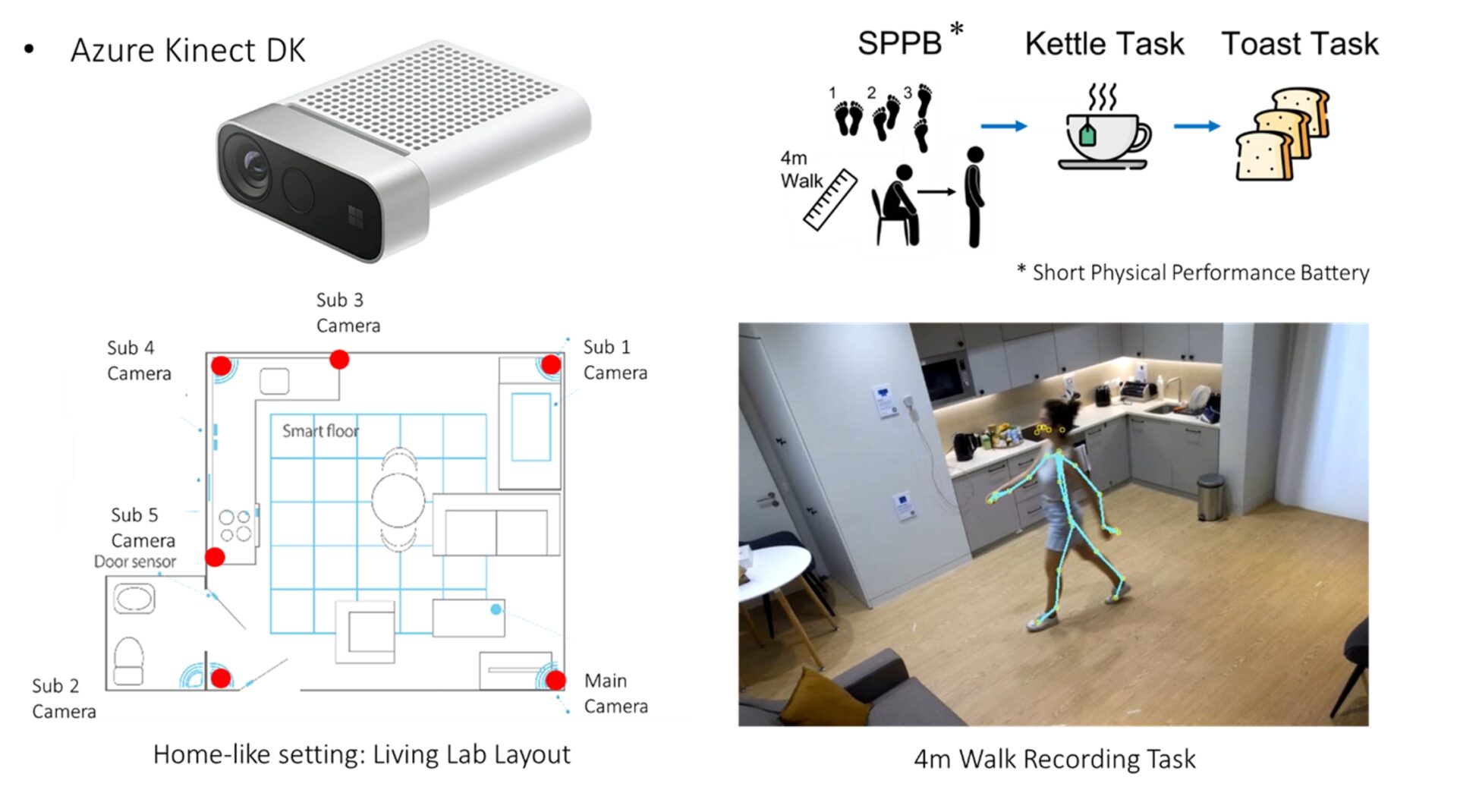
Markerless Motion Capture (MMC) can track full-body movement and posture to measure motor fluctuations during everyday activities, with the potential for deployment in people's homes, enabling continuous and objective measurement of PD’s severity [1,2]. In this project, which is part of the Living lab Study [3], we present a proof of concept for measuring symptom fluctuations and disease progression using passive sensors in domestic settings during activities of daily living (ADL). MMC using multiple Microsoft Kinect depth sensors have been used for tracking full-body movements of PD participants (N=12) as they perform mobility tests and ADLs in a living-lab environment. The participants performed the tasks once shortly after taking medication, while their symptoms were controlled (On state) and again a few hours later, before the next medication intake, when symptoms started to fluctuate (Off state). A case study participant attended seven visits over nine months. Features of their movement and posture were extracted and used to predict their UPDRS scores using multivariate linear regression models. The spine curvature and shoulder slope during walking, and centre of mass deviation during sit-to-stand were common features within the best subset for prediction in both data groups.
Feature selection was performed separately for the group and the case study. In the case study, a model based on features identified for this patient performed very well (R2 = 0.96) while using the features identified for the group reduced the model's performance (R2 = 0.76). Interestingly, when applying the case study's optimal features to the group, there was a substantial drop in R2 from 0.76 to 0.38.
Next, a classification between patients’ On and Off states based on their daily medication cycle was performed. A Support Vector Machine (SVM) model was used to train two models, one on the case study and another on the group. For the case study, their personalised model outperforms the group’s model with perfect classification vs AUC of 0.82. For the group data, the model performed well with an AUC of 0.85. Interestingly, the best outcomes for the case study were obtained using only features from the instructed tasks (4-metre walk and sit-to-stand), whereas the group obtained the best results by using only features from the ADLs (making tea and toasts).
This preliminary analysis suggests that MMC techniques could potentially be a reliable approach for monitoring and evaluating PD motor symptoms.By effectively addressing fluctuations, these techniques could offer valuable insights into optimizing medication dosages and tailoring patient care requirements, reinforcing the importance of personalized medicine in managing the evolution of this disease.