Introduction
Concerningly, 20% of women and 14% of men are estimated to be obese by 20301. The reproductive effects of maternal and paternal obesity have been investigated independently and include subfertility, fetal growth abnormalities, gestational disorders and adverse offspring health2,3,4. We hypothesise that two obese parents have divergent and compounded effects on male and female fetal growth associated with unique effects on placental morphology, transport and imprinted gene expression.
Aim
To assess the individual and combined role of parental obesity on fetal development, placental morphology and transport.
Methods
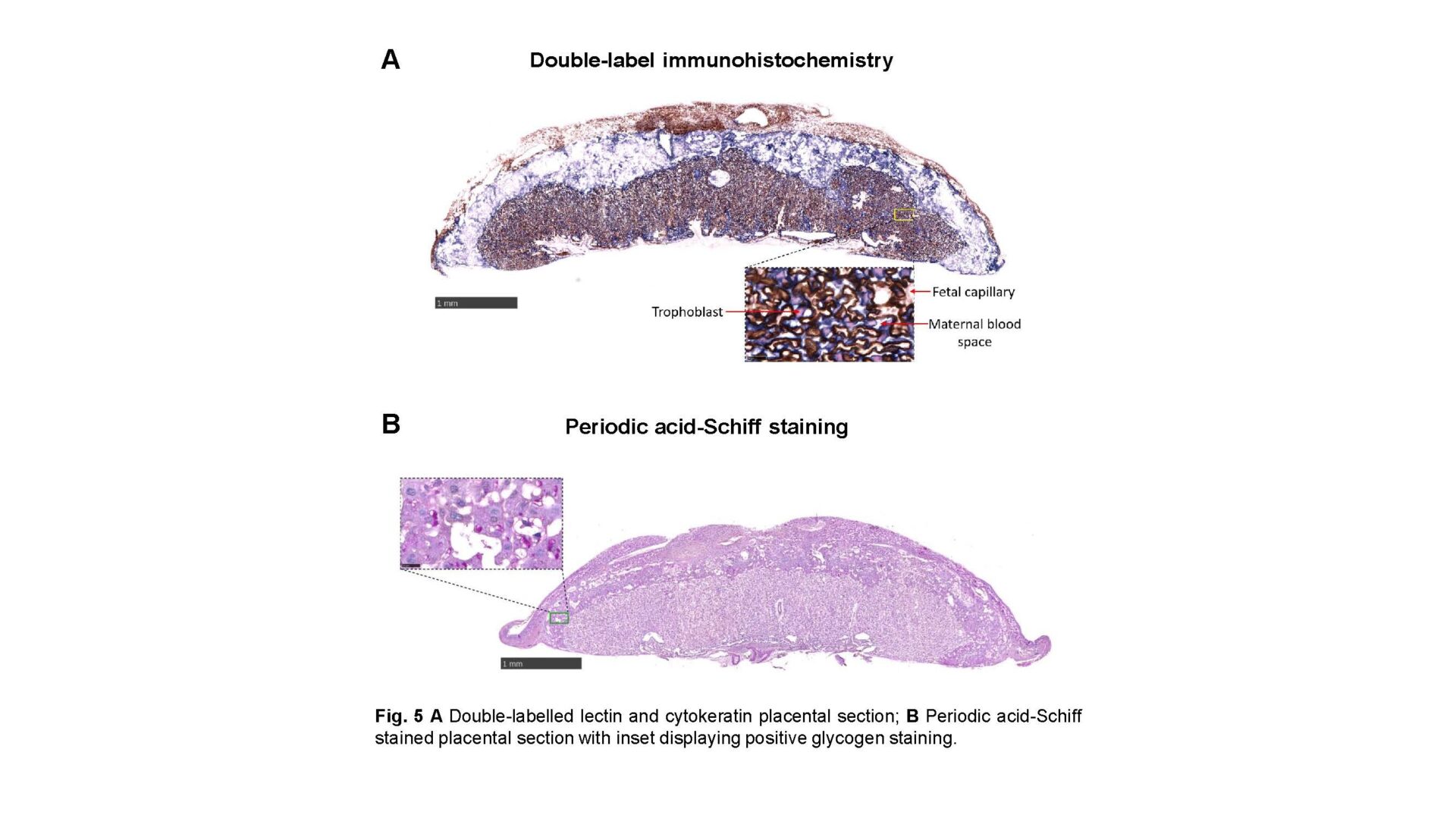
Female and male mice were fed a chow diet (11% fat, 7% sugar) or obesity-inducing high-fat/high-sugar diet (38% fat, 33% sugar). They were time-mated 5 weeks later, creating 4 pregnancy groups and females continued on the diet they consumed prior to mating. Stereological analyses on placentas (embryonic day 18.5; 4-6 fetuses/group) of gross morphology (point counting), interhaemal membrane thickness (length measurements), surface area for exchange (SA; cycloid arc intersections) and glycogen deposition (ImageJ analysis) were conducted following Haematoxylin and Eosin staining, Periodic acid-Schiff staining and double-label immunohistochemistry (4-6 replicates/group)5, respectively. Relative mRNA expression of imprinted genes and nutrient transporters was measured by RT-qPCR (6-8 fetuses/group; 1-2 fetuses/litter). To assess in vivo transplacental transport (>11 fetuses/group; 1-2 fetuses/litter), anaesthesia was induced in dams on embryonic day 18 by 90.9mg/kg Ketamine + 4.55mg/kg Xylazine via intraperitoneal injection (0.01mL/g body weight), followed by injection of radiolabelled glucose/amino acid analogues into the jugular vein.
Results (data quoted as mean±SEM)
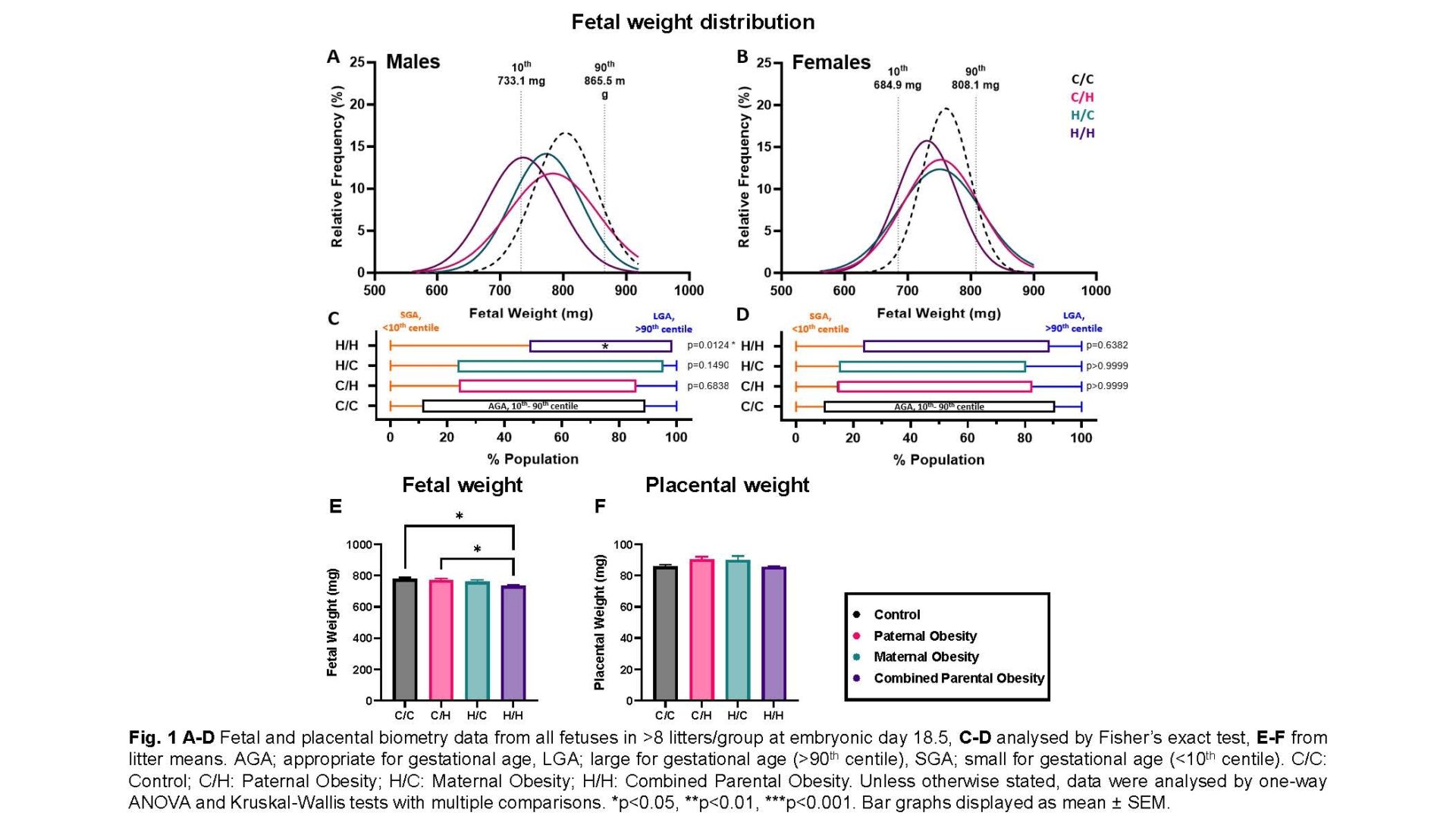
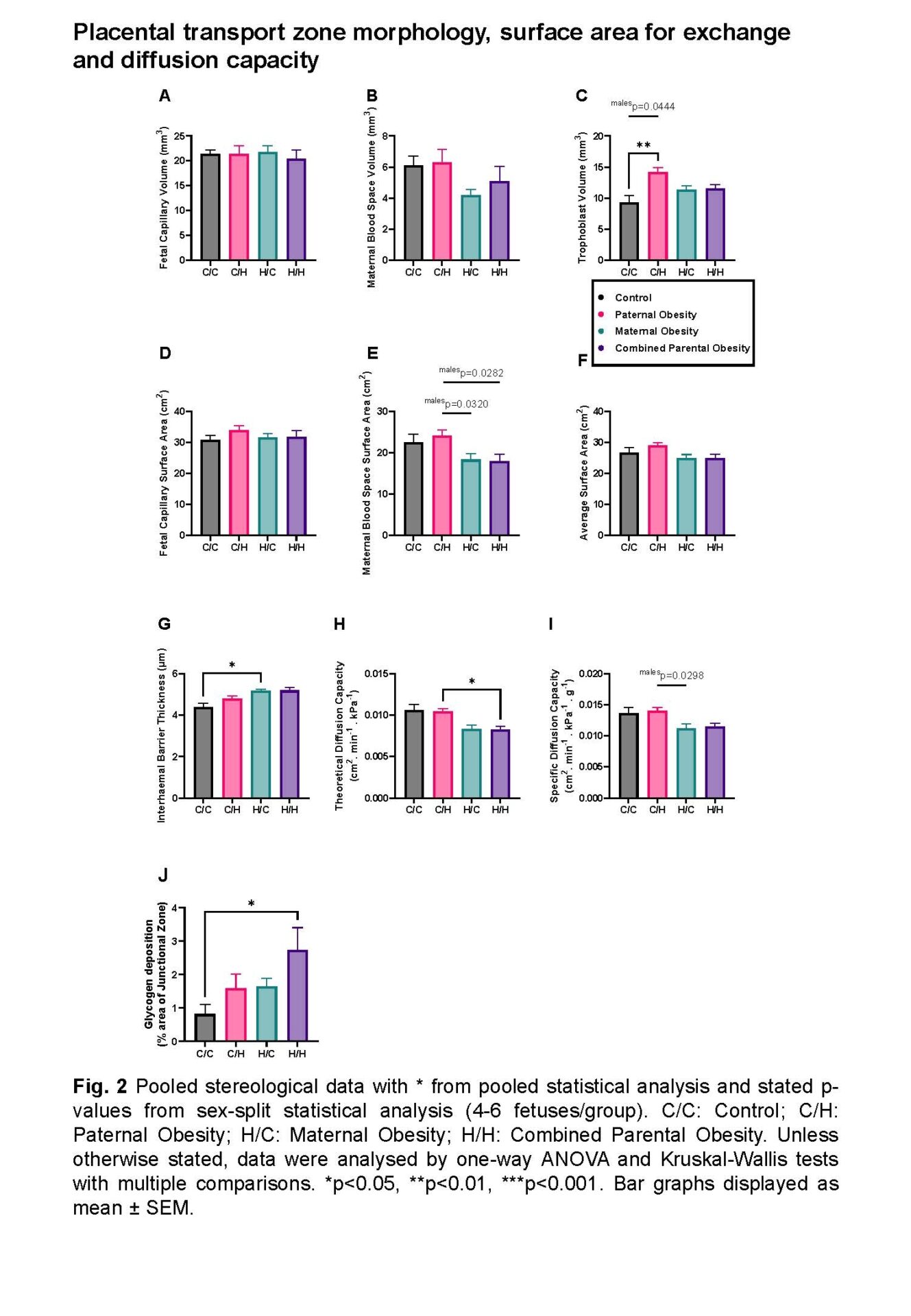
Male and female fetuses: Combined parental obesity reduced fetal weight relative to controls (734.0±8.202mg vs 777.8±10.54mg; p=0.0459; one-way ANOVA). Paternal obesity increased trophoblast volume (14.16±0.7277mm3 vs 9.274±1.126mm3; p=0.0031; one-way ANOVA) and maternal obesity increased interhaemal membrane thickness (5.177±0.06273μm vs 4.378±0.1919μm; p=0.0230; Kruskal-Wallis test) relative to controls. Combined parental obesity increased glycogen deposition relative to controls (2.731±0.6736% vs 0.8152±0.2813%; p=0.0242; one-way ANOVA) and reduced theoretical diffusion capacity relative to paternal obesity (0.008240±0.0003738cm2.min-1.kPa-1 vs 0.01047±0.0003094cm2.min-1.kPa-1; p=0.0452; Kruskal-Wallis test).
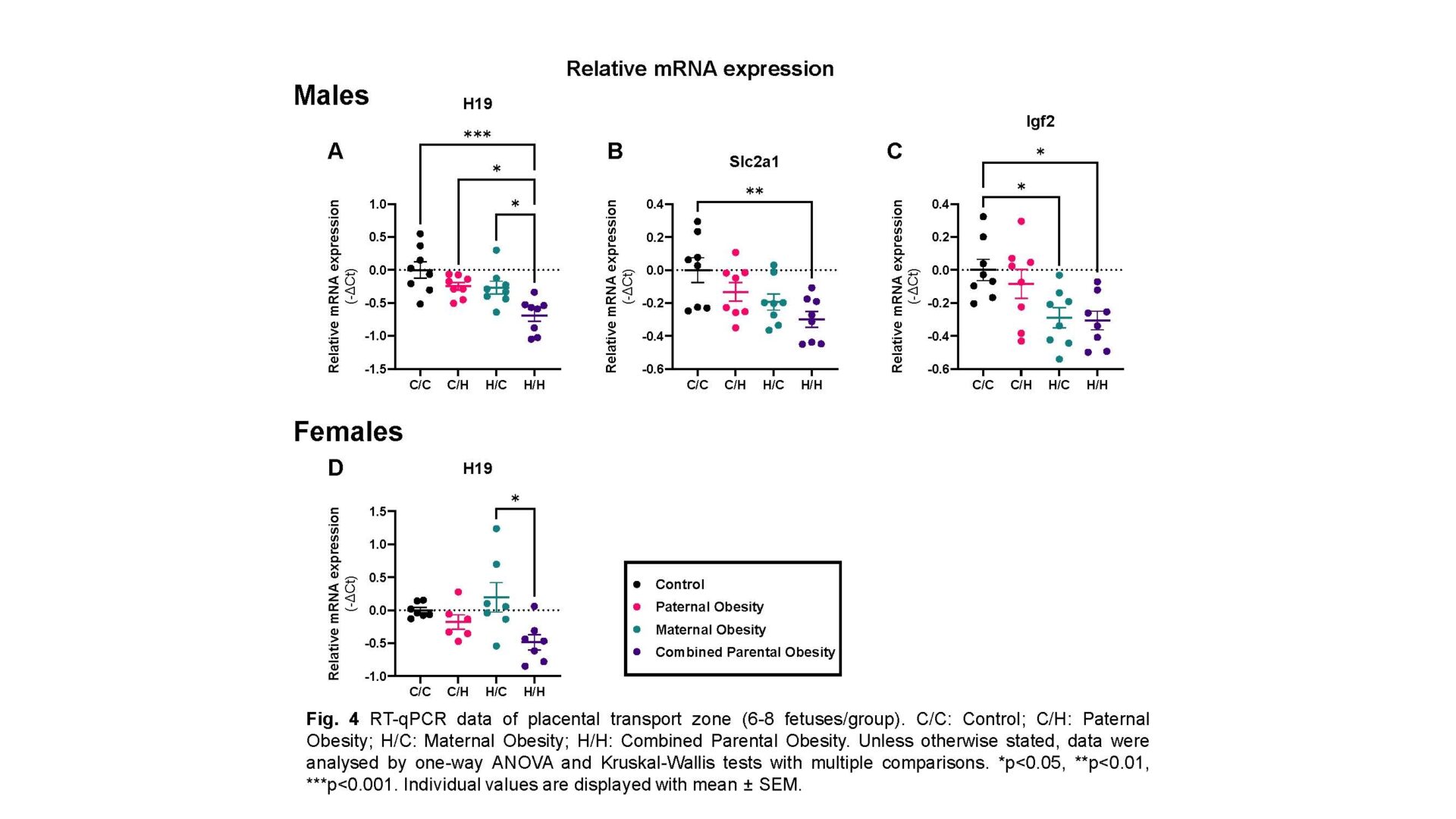
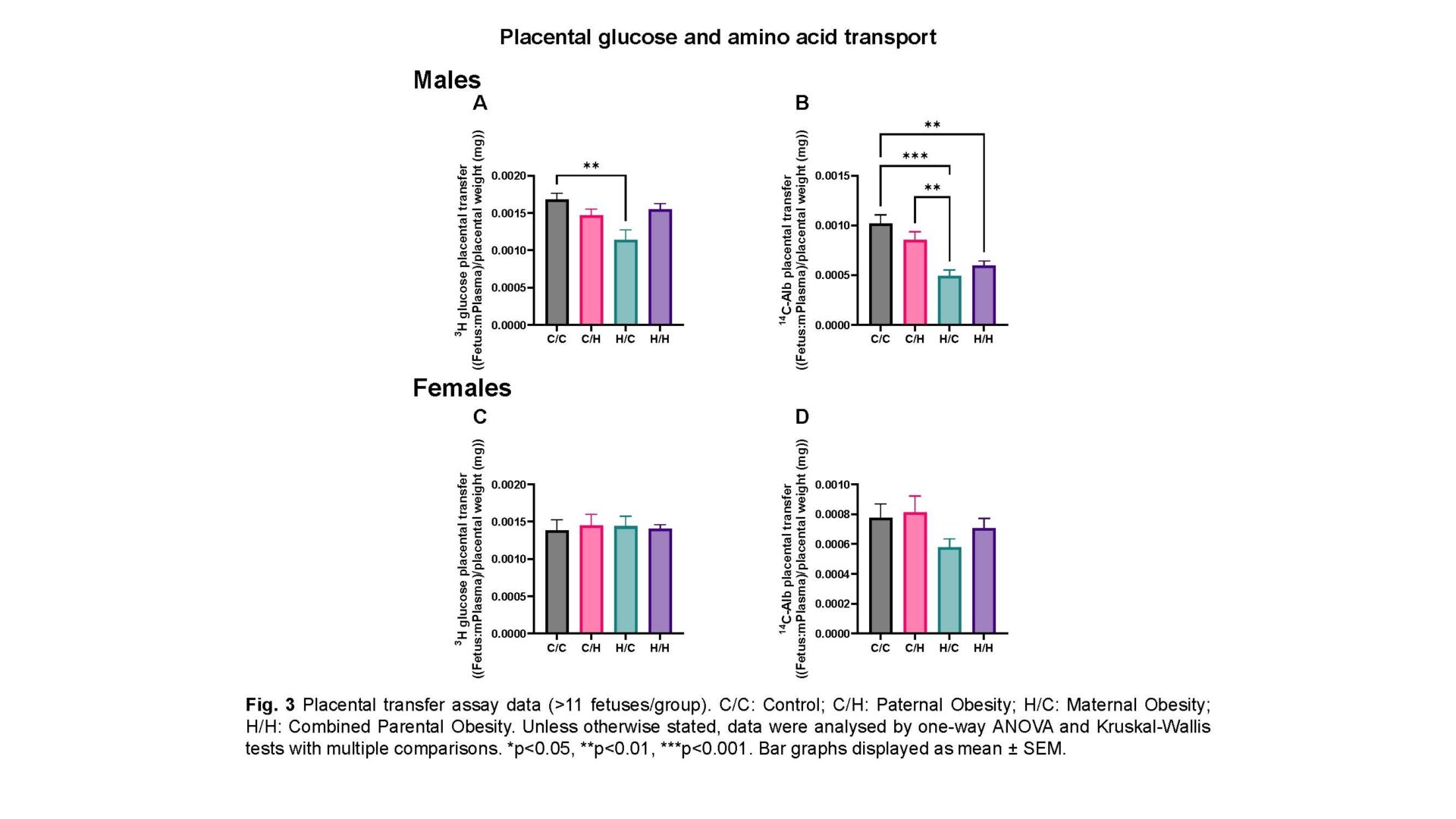
Male fetuses: Combined parental obesity reduced fetal weight (744.0±10.40mg vs 800.7±9.069mg; p=0.0225; one-way ANOVA), increased relative brain weight (0.07795±0.001488 vs 0.06748±0.003793; p=0.0214; Kruskal-Wallis test) compared to controls and decreased relative liver weight compared to paternal obesity (0.04370±0.0005922 vs 0.04917±0.001412; p=0.0470; one-way ANOVA), indicating asymmetric growth restriction. Maternal blood space SA was reduced in combined parental obesity (15.20±0.5187cm2 vs 23.95±1.668cm2; p=0.0282; one-way ANOVA) relative to paternal obesity. Combined parental obesity reduced relative H19 (-0.7055±0.1045 vs 0; p=0.0002; one-way ANOVA), Igf2 (-0.2911±0.06285 vs 0; p=0.0373; one-way ANOVA) and Slc2a1/GLUT1 (-0.2771±0.04968 vs 0; p=0.0158; one-way ANOVA) expression compared to controls. Reductions in glucose and amino acid placental transfer in maternal and combined parental obesity, respectively, were normalised by average SA.
Female fetuses: Fetal and placental weight/morphology were unaltered by parental obesity. H19 expression was reduced in combined parental obesity relative to maternal obesity (-0.4861±0.1163 vs 0.1954±0.2225; p=0.0089; one-way ANOVA).
Conclusion
Singular and combined parental obesity compromise fetal growth via sex-specific, parent-specific and combined effects on placental morphology, nutrient transporter and imprinted gene expression. Such changes would disturb transplacental transfer and highlight the reproductive ramifications of obesity/calorie-dense diets in both parents.