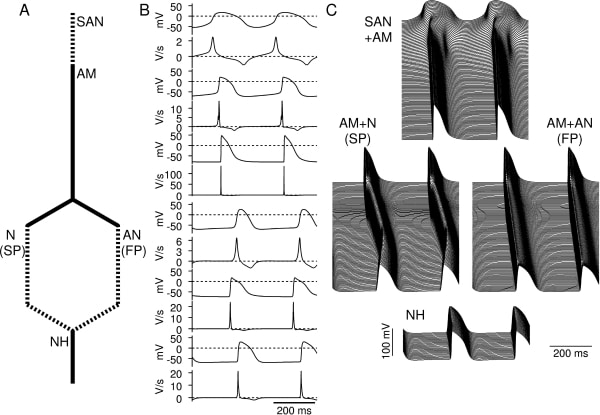
Mathematical models can be a repository of knowledge as well as important research and teaching tools. Although models of the action potential have been developed for most regions of the heart, there is no biophysically-detailed model for the atrioventricular node (AVN) and the aim of this study was to develop such a model. We have developed models of the action potential in single atrio-nodal (AN), nodal (N), and nodal-His (NH) cells of the rabbit AVN based on published electrophysiological data. Using these single cell models, together with single cell models of the sinoatrial node (SAN) (Zhang et al. 2000) and atrial muscle (AM) (Lindblad et al. 1996), we have developed a simplified one-dimensional (1D) model including the SAN and AVN (Fig. 1A). This model consisted of a string of 50 SAN cells (central and peripheral SAN cells) connected to a string of 150 atrial cells. The atrial cells are connected to two parallel pathways, a slow pathway (SP) (a string of 50 atrial cells and 150 N cells) and a fast pathway (a string of 50 atrial cells and 150 AN cells). Both pathways are connected to a string of 50 NH cells. Neighbouring cells are coupled by a coupling conductance. The single cell models have the same action potential characteristics (maximum diastolic potential, upstroke velocity, amplitude, and action potential duration) and refractoriness as observed in experiments (Fig. 1B). Using the 1D model, we simulated action potential propagation. Under normal conditions, action potentials were initiated in the centre of the SAN and then propagated through the atrium and AVN (Fig. 1C). Using the 1D model excluding the SAN, we analysed the characteristics of the AV node. After stimulation of the atrial muscle, the action potential propagated along the parallel slow and fast pathways, but conduction along the fast pathway was faster than conduction along the slow pathway. After premature stimulation, conduction along the fast pathway was blocked, and then AVN reentry could occur. After slow pathway ablation or partial block of the L-type Ca2+ current, AVN reentry was abolished. During atrial fibrillation, the AV node limited the number of action potentials transmitted to the ventricle. Pacemaker activity arose from the slow pathway in the absence of SAN pacemaking. These results are consistent with experimental data. In conclusion, we have developed the first biophysically-detailed model of the AVN and it shows many of the typical physiological and pathophysiological characteristics of the tissue. The model can be used as a tool to analyse the complex structure and behaviour of the AV node.
University of Manchester (2007) Proc Physiol Soc 8, PC29
Poster Communications: Mathematical model of the atrioventricular node
S. Inada1, J. C. Hancox2, H. Zhang1, M. R. Boyett1
1. University of Manchester, Manchester, United Kingdom. 2. University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Fig. 1. Simulated action potentials in 1D model from SAN to AVN
Where applicable, experiments conform with Society ethical requirements.