Light decreases photoreceptor Ca2+i by reducing its influx through the outer segment conductance while allowing continued efflux via Na+-Ca2+-K+ exchange. However, presently available fluorescent Ca2+ indicators require an excitation intensity sufficiently high as to bleach almost all of the photopigment in amphibian or reptile photoreceptors, allowing only a single measurement. We have resolved this problem by using instead UV-sensitive zebrafish cones, which are most sensitive at 365 nm and are ~105 times less sensitive to the 514 nm light of our laser spot microscope.
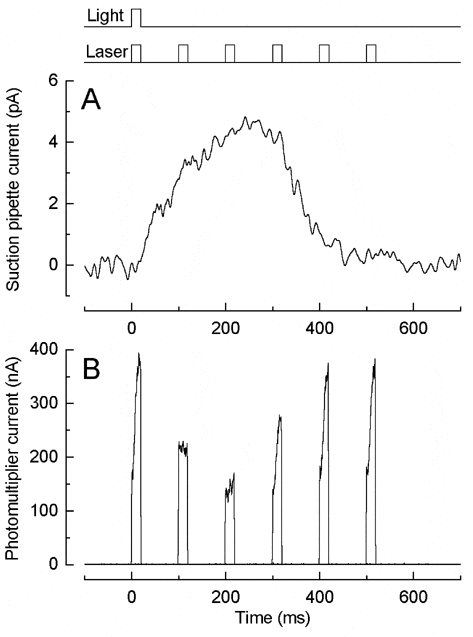
Zebrafish were killed according to Schedule 1 by cranial concussion followed by decapitation and pithing. Fluo-4 was loaded as its AM ester into the outer segment of an isolated UV-sensitive cone whose inner segment was held by a suction pipette, and excited by an argon ion laser (Sampath et al. 1998). The outer segment was exposed to a near-saturating 405 nm flash (Fig. 1A) and then at a variable time thereafter to a 514 nm laser flash (Fig. 1B), calculated to bleach less than 0.02 % of the photopigment. By repeating this protocol with different time intervals, the time course of dye fluorescence during the flash response can be determined from a single cell.
Laser flashes delivered early or late in the light response exhibited a rapid rise in fluorescence, which was not present for those delivered at the response peak. When the laser flash was delivered in a 0 Ca2+/0 Na+ solution designed to minimise Ca2+ fluxes this fast initial rise (t = 6.7 ± 0.7 ms, n = 8 cells) accounted for 0.31 ± 0.11 (mean ± S.E.M.) of the fluorescence. However, when cells were pre-incubated with 100 µM BAPTA/AM its amplitude (0.28 ± 0.06, n = 8 cells) and kinetics (t = 8.3 ± 0.95 ms) were not significantly affected (Student’s t test, 5 % level), suggesting that it represented a change in dye properties instead of a change in Ca2+i. In contrast, at later times in 0 Ca2+/0 Na+ solution, a 15 ± 3 % rise in fluorescence was observed with a slower (1-2 s) time course which was abolished by BAPTA incorporation, suggesting that light may induce a release of Ca2+ in zebrafish cones as in salamander rods (Matthews & Fain, 2001).
This work was supported by The Wellcome Trust and the NEI of the US NIH.