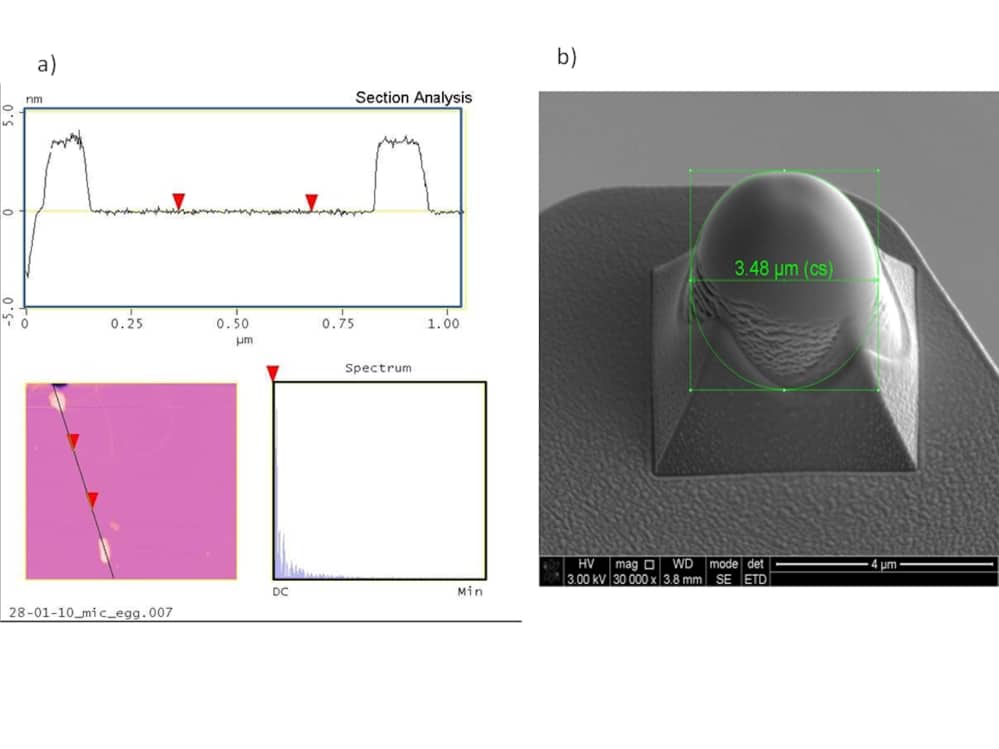
This study aims to understand the structure and mechanical characteristics of bilipid membranes for an artificial mechanoreceptor. This includes defining Young’s modulus and bending rigidity. Three types of lipid molecule, L-α-phosphatidylcholine (Egg-PC), 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-didodecanoyl-sn-glycero-phosphocholine ( DLPC) (Avanti Polar Lipids, Inc.,) are being used for comparison in both vesicle and bilipid planar forms. These were chosen since they have different lipid tail lengths such that gel and fluid phases can be compared (Goertz et al.,2009). The bilipid membranes have been fabricated on supporting mica substrates such that their dynamic and static stress responses can be studied using Atomic Force Microscopy. Nanometre unilamellar vesicles were prepared by dissolving lipid powder in chloroform, evaporating the organic solvent using a dry nitrogen stream and then drying in a desiccator connected to a rotary vacuum pump. The resulting lipid film was hydrated and sonicated to produce vesicles with a range of diameters. These were re-suspended by stirring them in an aqueous buffer solution (0.5 mg/ml) and sonicated to clarity. The resulting vesicles were then deposited onto a mica surface within a water filled fluid cell of an Atomic Force Microscope (Veeco Multimode E®) (AFM) at room temperature. The concentration and fluid cell flow rate determines the resulting formation. i.e. vesicle or planar bilayer. A scanned image of a planar bilayer of Egg-PC on a mica substrate in deionised water is shown in Figure 1a. The section analysis displays a membrane thickness of 3.8 nm +/-0.6nm, confirming the presence of a bilayer. This is consistent with the literature where values range from 3.9nm+/-0.4nm to 5nm+/-1nm (Liang et al 2004, Reviakine et al., 2000). A similar technique was applied using DSPC and DLPC. High yield fabrication of reliable bilayer structures is needed for sensor development with yields presently as low as 33% (Pioufle et al., 2008). To measure the bending rigidity of vesicle structures, the AFM is used to produce force-distance curves. A Hertz contact model assuming a spherical shape for both the tip and vesicle (Liang et al., 2004) is then applied. A softer rounded AFM tip has been fabricated by locally depositing platinum on top of standard silicon nitride cantilevers in a Focussed Ion Beam (FIB) system (Figure 1b). This enables a Hertz model to be applied to planar membranes (Lebedev et al., 2009) to measure Young’s modulus. To conclude, methods to measure the mechanical and structural properties of bilipid structures have been developed. However, fabrication yield needs to be improved to produce repeatable structures to develop a viable sensing mechanism.
Durham University (2010) Proc Physiol Soc 21, PC31
Poster Communications: Mechanical Properties of Bilipid Layers for a Bioinspired Mechanoreceptor
X. Duan1, S. Arnold1, M. C. Rosamond1, J. Atherton1, A. J. Gallant1, S. Pyner2, S. Johnstone1
1. School of Engineering and Computing Sciences, Durham University, Durham, United Kingdom. 2. Biological and Biomedical Sciences, Durham University, Durham, United Kingdom.
View other abstracts by:
Figure 1: a) Section view, plan view and spatial spectrum of Egg-PC planar layer partially coating mica substrate b) Platinum Coated Silicon Nitride AFM tip for planar membrane testing
Where applicable, experiments conform with Society ethical requirements.