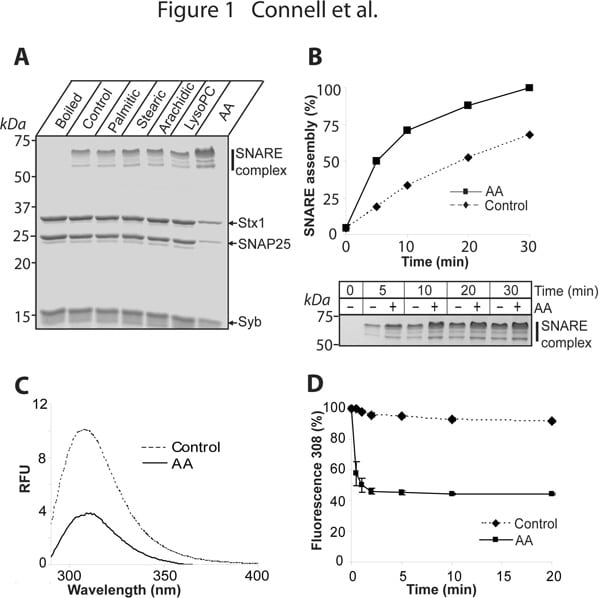
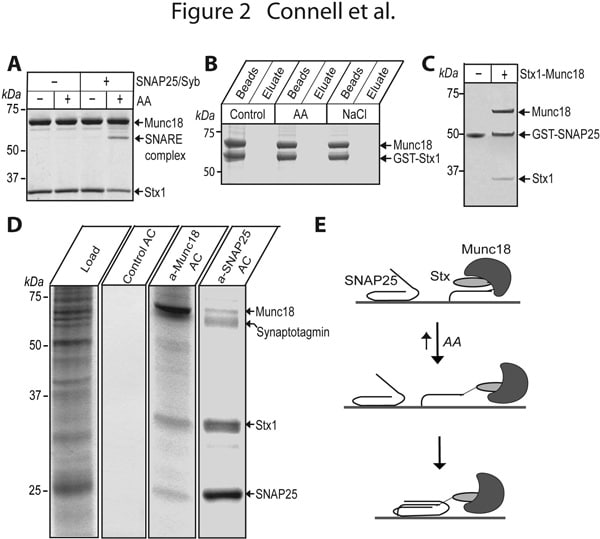
The ubiquitous process of membrane fusion in eukaryotes is executed by a host of essential conserved factors, including membrane associated SNARE proteins such as syntaxins. SNARE complex formation brings membranes into close apposition for fusion. The lipid metabolism of the surrounding bilayer, and highly conserved cytosolic components such as Munc18, are also important. The interaction of Munc18 with syntaxin is essential for exocytosis. Knock-out of Munc18 results in dramatically decreased secretion (Verhage et al, 2000) yet paradoxically, initial in vitro experiments suggested that the syntaxin/Munc18 interaction is incompatible with SNARE assembly. It was recently found that Munc18 inhibition of neuronal syntaxin1 can be overcome by arachidonic acid (Rickman and Davletov, 2005), suggesting that this common second messenger disrupts syntaxin/Munc18 association. We now show that arachidonic acid can stimulate syntaxin1 in the absence of Munc18 (Figure 1). Although arachidonic acid relieves inhibition of Munc18-bound syntaxin (Fig2A), it cannot dissociate this syntaxin1/Munc18 complex (Fig2B). Upon arachidonic acid-induced syntaxin1/SNAP25 association, Munc18 remains attached to syntaxin1, and a Munc18/syntaxin/SNAP25 complex is formed (Fig2C). We show for the first time that neuronal SNAREs can be isolated from brain membranes assembled with endogenous Munc18 (Fig2D,E) consistent with Munc18’s role in downstream vesicle docking and fusion events (Toonen et al, 2006).
Life Sciences 2007 (2007) Proc Life Sciences, PC299
Poster Communications: Mechanism of arachidonic acid action on syntaxin/Munc18
E. Connell1, F. Darios1, N. Gatsby1, C. Rickman1, B. Davletov1
1. Neurobiology Division, MRC-LMB, Cambridge, United Kingdom.
View other abstracts by:
Fig1. Action of arachidonic acid (AA) on syntaxin1 (Stx1). (A) SNARE reactions in the presence of 100 µM saturated palmitic (C16:0) stearic (C18:0) arachidic (C20:0) acids brain lysophosphatidylcholine (LysoPC) and unsaturated AA (20:4). Coomassie-stained gel. (B) AA-induced acceleration of SNARE assembly. (C) (D) Rapid AA-triggered change in syntaxin1 intrinsic fluorescence (excitation 280 nm).
Fig2. Interaction of native Munc18 with syntaxin1 persists following arachidonic acid-induced SNAP25 engagement. Coomassie-stained gels. (A) AA allows SNARE assembly with syntaxin1/Munc18. (B) GST-syntaxin1 association with brain Munc18 is not disrupted by 100 µM AA or 1 M NaCl. (C) Incubation of GST-SNAP25 with syntaxin1/Munc18 binary complex in the presence of 100 µM AA leads to pull-down of both syntaxin1 and Munc18. (D) Munc18 co-purifies with syntaxin1/SNAP25 from brain extract (load) during preparative affinity chromatographies (AC) using anti-Munc18 or anti-SNAP25 BrCN beads. (E) AA allows SNAP25 engagement by syntaxin without Munc18 dissociation.
Where applicable, experiments conform with Society ethical requirements.