In electrically stimulated cells, sevoflurane (Sevo) initially decreases contraction followed by partial recovery during the exposure. On removal of Sevo, contractions transiently increase above control (Davies et al. 2000). Hannon & Cody (2002) reported that Sevo increased sarcoplasmic reticulum (SR) Ca2+ content in ferret ventricle (via inhibition of sarcolemmal Ca2+-ATPase), which could underlie the increase in contractions on wash-off. However, Davies et al. (2000) reported no change of SR Ca2+ content at the end of an exposure to Sevo in rat ventricle, tissue where the sarcolemmal Ca2+-ATPase plays a much smaller role in Ca2+ regulation. We have investigated other possibilities which could contribute to the inotropic profile of Sevo including changes in myofilament Ca2+ sensitivity and the sensitivity of the SR Ca2+ release process.
Wistar rats (200-250 g) were humanely killed, the heart removed and ventricular myocytes isolated by enzymatic dispersion. Contraction and intracellular Ca2+ (with fura-2) were recorded optically in both unstimulated cells and paced cells (1 Hz). Cells were superfused with a normal Tyrode solution (1 or 2 mM Ca2+) and exposed to 0.6 mM Sevo for a period of 1 or 4 min at 30 °C. Data are presented as means ± S.E.M. and Student’s paired t test was used to assess significance.
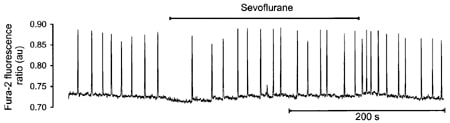
In unstimulated cells, raising bathing Ca2+ to 2 mM induced regular spontaneous Ca2+ transients and contractions (frequency of 0.09 ± 0.01 Hz, n = 8; see Fig. 1). During the first 30 s of a 4 min exposure to Sevo the frequency of spontaneous Ca2+ release was significantly reduced (to 0.03 ± 0.01 Hz, P = 0.024) but returned to control (0.08 ± 0.01 Hz) over the next 30-60 s suggesting a transient decrease in the sensitivity of the SR Ca2+ release process. Removal of Sevo significantly increased spontaneous activity (to 0.1 ± 0.01 Hz, P = 0.02) before returning to control (0.075 ± 0.01 Hz) over the next 30 s, similar to that observed with tetracaine (Overend et al. 1997). However, no significant changes in the amplitude of spontaneous Ca2+ transients were observed either before, during or after exposure to SEVO.
Contraction and Ca2+ transients were recorded in electrically stimulated cells (1 Hz) before, during and following a 1 min exposure to sevoflurane. From plots of shortening vs fura-2 Fr from individual twitches, regression lines were fitted to the final phase of relaxation, the slope of which provides an index of myofilament Ca2+ sensitivity (Spurgeon et al., 1992). In 12 cells, the control slope was 121.1 ± 7.2 µm (Fr unit)-1. At the point of maximum negative inotropy of Sevo (after ~5 s) the slope was significantly (P < 0.001) reduced (to 117.1 ± 6.1 µm (Fr unit)-1) but recovered partially during the exposure (slope 118.5 ± 6.7 µm (Fr unit)-1). On wash-off, the slope was significantly increased above control (to 122.6 ± 7.3 µm (Fr unit)-1; P < 0.001) before returning to its original value (121.5 ± 7.2 µm (Fr unit)-1).
These data suggest that changes in the sensitivity of (i) the SR Ca2+ release process and (ii) the myofilaments to Ca2+ contribute to the negative and positive inotropic actions of sevoflurane.
This work was supported by the British Heart Foundation and The Medical Research Council.