The connexin (Cx) family of proteins form gap junctional channels that allow intercellular transfer of small molecules and ion currents. Some genes of the Cx family, particularly Cx26 (GJB2), Cx31 (GJB3) and Cx30 (GJB6) have been implicated in hereditary deafness. Although most of these mutations are associated with a complete loss of function, some of them do retain the ability to form functional channels (Ressot et al. 1998). Cx26 and Cx30 are abundantly expressed amongst supporting cells of the organ of Corti in the cochlea (Lagostena et al. 2001).
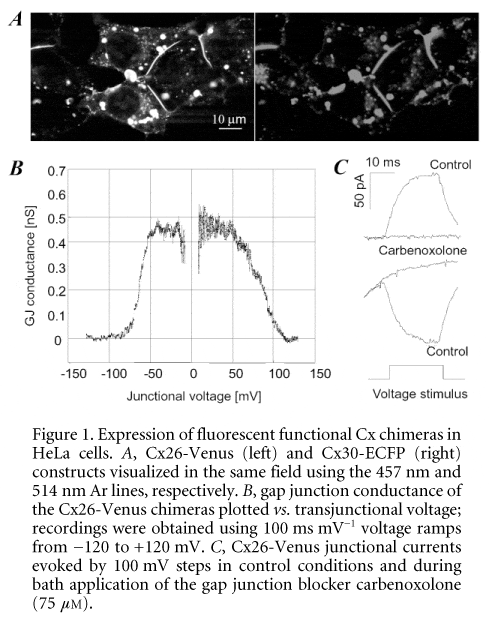
In an effort to unravel the molecular mechanisms underlying the development of a pathological phenotype in the presence of mutated function channels, we molecularly engineered fluorescent chimeric constructs formed by Cx26 and Cx30 each genetically fused to the cyan (ECFP) and yellow (Venus) colour variants of the green fluorescent protein (GFP). The cDNA of Cx26 and 30 was amplified from genomic DNA deleting the authentic Cx stop codons. The 3Ô end of Cx cDNAs was then fused to the 5Ô end of the GFP mutant cDNA through a linker coding for six amino acids (DPPVAT) and cloned in pcDNA3.1zeo+ transfection vectors (Invitrogen, Carlsbad, CA, USA). Transient transfection was performed on cultures of communication-deficient human cervical carcinoma (HeLa) cells grown to 50-75 % confluency in Opti-MEM medium (Invitrogen) containing lipofectamin (Invitrogen) and 1 mg of plasmid DNA. Living cells co-transfected with Cx26-Venus and Cx30-ECFP constructs were illuminated by the 457 nm (ECFP) and 514 nm (Venus) lines of the Argon laser in a BioRad 2100 confocal microscope with emission filter settings that minimized the bleed-through of the cyan and yellow channels. Image analysis revealed extensive co-localization of the two Cx types in the same gap junction plaques formed by constructs which appeared correctly targeted at the plasma membrane of contact regions between adjacent cells (Fig. 1A).
HeLa cells transfected with the Cx26-Venus and Cx30-Venus constructs were additionally used to obtain dual patch clamp recordings. Cell pairs visually identified as connected by fluorescent plaques revealed that these gap junctions contained functional channels, which also exhibited the correct dependence of junctional conductance on transjunctional voltage (Fig. 1B) and were blocked by application of CO2 (100 %) or carbenoxolone (75 mM; Fig. 1C). Voltage gating properties were investigated by subjecting one of the two cells to ramps of potential from the holding potential (0 mV) to ±120 mV and recording the whole-cell current in the unstimulated cell. Peak conductances (measured around 0 mV) were 0.55 ± 0.15 nS and 3.8 ± 1.2 nS in Cx26 (n = 3 pairs) and Cx30 (n = 4 pairs) transfectants, respectively. When measured with ramp speeds in the range 100-200 ms mV-1, conductance half-inactivation voltages were (in absolute value) 55 ± 3 mV (Cx26) and 38 ± 2 mV (Cx30). These preliminary results indicate that the combination of molecular biology, fluorescence microscopy and electrophysiology may pave the way to the identification of Cx functional deficits relevant to hearing impairment.
This work was supported by grants from the INFM (R.L. TSB2) and MIUR to F.M. and Tullio Pozzan. M.B. and V.P. were supported by fellowships from the IRCSS S.G.R.