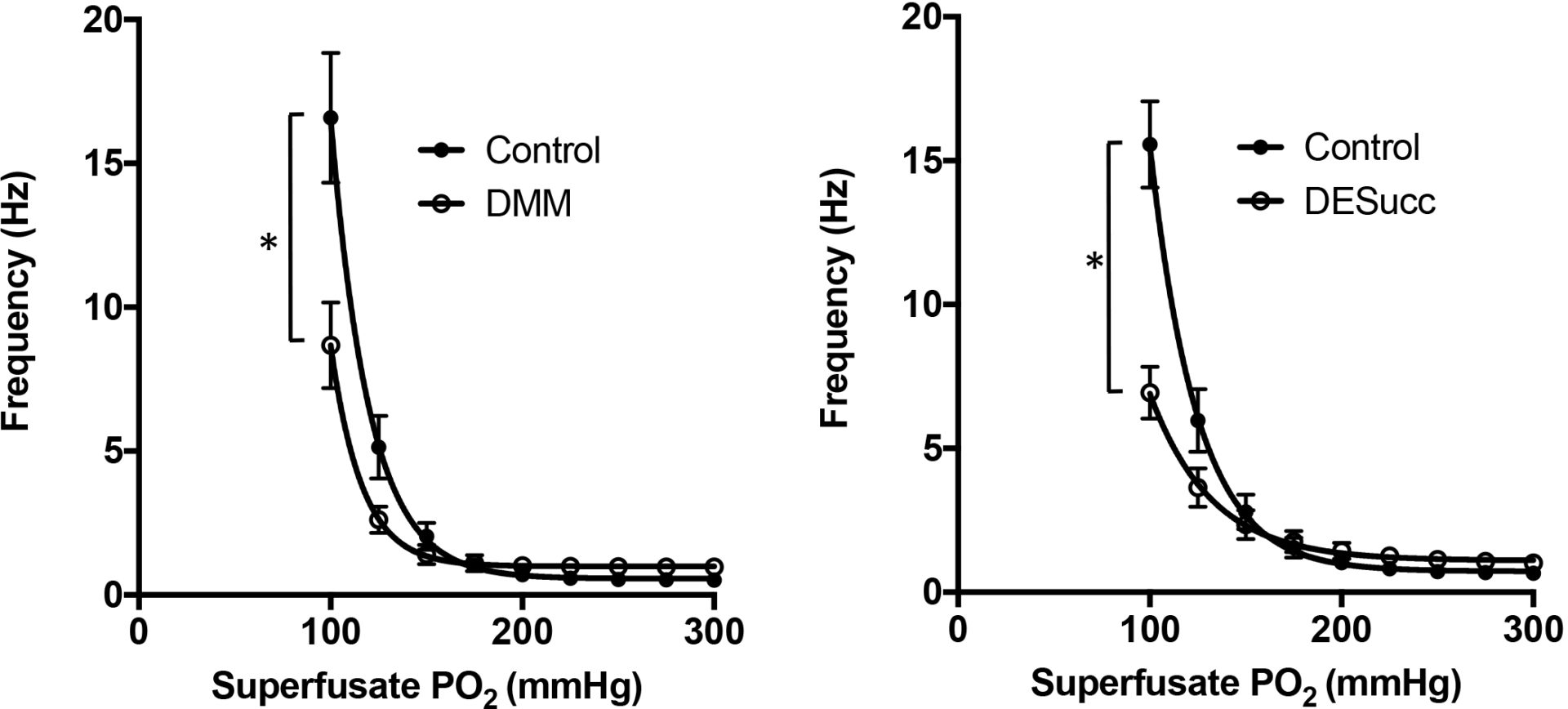
The mammalian carotid body (CB) is a unique peripheral chemoreceptor that responds to acute hypoxia well before the metabolism of other cell types is impaired [1]. However, the specific O2 sensing mechanism in the CB remains highly contested. Recently it has been suggested that succinate metabolism at mitochondrial complex II is necessary to cause CB excitation during hypoxia [2]. This has been countered by the finding that CBs with heterozygous Sdhd knock out display an augmented, rather than depressed basal activity and retain sensitivity to hypoxia [3]. Thus, the role of CB succinate metabolism and downstream signalling during hypoxia requires further investigation. The aim of this current study is to evaluate if CB chemoafferent activity can be modified by changes in succinate metabolism during normoxia or hypoxia. All animal procedures were approved and carried out in line with the current Home Office (UK) and University of Birmingham guidelines on ethical use of animals. CBs were isolated from adult male Wistar rats (150-250g) and recordings of single fibre chemoafferent activity was performed as previously described [4]. Data was expressed as mean ± S.E.M and significance determined with paired t-test or one-way repeated measures ANOVA and taken as P<0.05. Application of cell permeable diethyl succinate (DESucc; 1-10mM) caused a rapid, reversible and concentration dependent increase in chemoafferent activity (e.g. Control 0.5±0.1Hz, 10mM DESucc 2.4±0.4Hz (P<0.05)). The peak elevation in chemoafferent activity induced by DESucc measured approximately 15% of that caused by exposure to severe hypoxia. The rise in chemoafferent activity evoked by 5mM DESucc (1.8±0.2Hz) was significantly reduced by application of 10mM di-methyl malonate (DMM; 0.4±0.2Hz, P<0.05); a mitochondrial complex II inhibitor, or by mitoTempo (20mM; 0.8±0.3Hz, P<0.05); a mitochondrial antioxidant. Interestingly, 5mM DESucc also reduced the chemoafferent response to hypoxia (e.g. at superfusate PO2 of 100mmHg: Control 15.5±1.5Hz, DESucc 6.9±0.9Hz, P<0.05, Figure 1 right panel). Application of 10mM DMM caused a small but significant increase in basal chemoafferent activity and depressed the CB response to hypoxia without completely abolishing it (e.g. at superfusate PO2 of 100mmHg: Control 16.6±2.2Hz, DMM 8.7±1.5Hz, P<0.05, Figure 1 left panel). These results suggest that endogenous mitochondrial succinate metabolism in the CB acts to repress basal CB chemoafferent activity. Furthermore, whilst we support the idea that succinate metabolism is required to confer the full CB chemoafferent response to hypoxia, we do not think it is not the sole mediator. Interestingly, excessive succinate metabolism acts to dampen the CB hypoxic sensitivity. Future experiments are required to see if these effects are mediated at the level of the glomus cell or nerve ending.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, PC017
Poster Communications: Mitochondrial succinate metabolism is important in setting carotid body chemoafferent activity in normoxia and hypoxia
A. Coney1, C. J. Ray1, A. Alzahrani1,3, P. Georgiadou1, A. Swiderska1, N. Batis2, P. Kumar1, A. P. Holmes1
1. Institute of Clinical Sciences, University of Birmingham, Birmingham, United Kingdom. 2. Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom. 3. Respiratory Care Department, College of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia.
View other abstracts by:
Changes in mitochondrial succinate metabolism modify the carotid body chemoafferent response to hypoxia. Inhibiting mitochondrial complex II with dimethyl malonate (DMM; 10mM-left) reduces but does not abolish the carotid body chemoafferent elevation during hypoxia. Increasing succinate metabolism, achieved through application of diethyl succinate (DESucc; 5mM-right), also decreases the response to hypoxia.
Where applicable, experiments conform with Society ethical requirements.