Experimental findings on mechanoelectric feedback in isolated myocardium and single cardiomyocytes have yielded a wide variety of stretch-induced changes in action potential duration (APD).
Earlier mathematical modelling has shown that APD responses may depend on stretch timing and the predominant mechano-sensitive mechanism activated. Thus early activation of stretch-activated ion channels (SACs) shortens APD, while late activation prolongs it. The opposite effects may be observed if the predominant target mechanism is troponin C (TnC) affinity for Ca2+ (Kohl et al. 1998).
To extend applicability of the model to contraction-related mechanical modulation of cardiomyocyte electrophysiology during various modes of contraction, we developed the Ekaterinburg/Oxford model which uses an improved description of cellular Ca2+ handling and mechanics, namely co-operative modulation of Ca2+ dissociation from TnC (Garny et al. 2001). This allowed us to relate changes in APD to length-dependent differences in Ca2+ dissociation from TnC and knock-on effects on Ca2+ transient and Na+-Ca2+ exchange current (iNaCa). The model simulated a range of stretch effects, but not cross-over of repolarisation and late APD prolongation, or afterdepolarisation-like events during large stretch. Only then did the model produce these effects when we also included SAC currents (iSAC).
We developed a routine that allows quantitative assessment of the influence of stretch-induced change in individual currents on AP shape. This is based on calculation of the cumulative effect (integral) of the difference Δik in the kth current during mechanical stimulation and control. The integrals contribute to AP change as in:

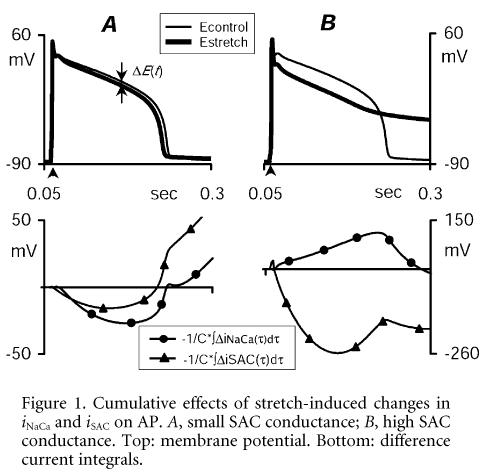
Comparison of integrals allows elucidation of the relative significance of individual currents for observed total AP change. Thus in the case of a small SAC conductance (0.03 nS for both control sarcomere length 1.9 mm and 5 % stretch), stretch-induced APD shortening is caused by a repolarising effect on AP of change in both iNaCa and iSAC (Fig. 1A). In the case of high SAC conductance (0.66 nS for 5 % stretch), the cross-over of repolarisation is driven by the opposite effects of these current changes (Fig. 1B).
This work is supported by The Wellcome Trust and the Russian Foundation for Basic Research.