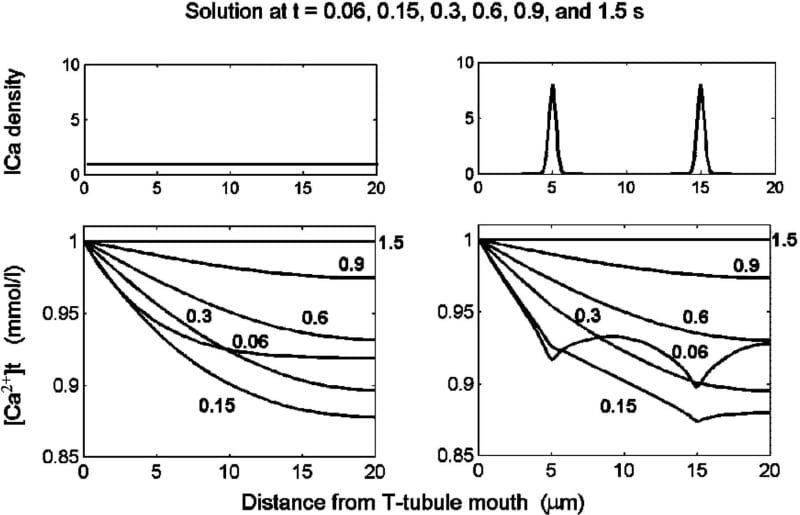
To calculate the spatial and temporal distribution of [Ca2+] within the transverse tubules (t-tubules) of cardiac myocytes, we have developed a model described by partial differential equations. Ca2+ diffusion, and Ca2+ binding at the t-tubule membrane and/or in the t-tubule lumen were modelled for cylindrical (254 nm diameter) t-tubules of finite length. When the Ca2+ binding rate constants were set to 2 mM-1 s-1 (kon), 10-2 s-1 (koff) and the diffusion coefficient to 8×10-6 cm2 s-1, the model responded to a sudden increase of bulk extracellular [Ca2+] from 0 to 1 mmol/l in a way that reproduced the wave-like propagation of [Ca2+] along the t-tubules described by Blatter and Niggli (1998). The velocity of propagation decreased markedly with increased t-tubular length, varying between 5 and 60 μm s-1 in the middle of 5 to 30 μm long t-tubules. To study the effect of Ca2+ channel distribution (and thus of ICa-density) along the t-tubules, two separated clusters of Ca2+-channels were considered (Fig. 1, upper right panel). The non-uniformity in ICa-density was reflected in non-homogeneous Ca2+-depletion along the t-tubule during activation of ICa, which was inactivated with a time constant of 50 ms (Fig. 1, lower right panel). Due to the Ca2+-buffering, the initial effect of Ca2+-channel localization was damped out rapidly: the initial irregularities in Ca2+ depletion were prominent 60 ms after activation of ICa, very small after 150 ms and absent after 300 ms. For comparison, when assuming uniformly distributed ICa transferring the same electrical charge across the t-tubular membrane (Fig. 1, left panels), the model predicts, at all times, a monotonous increase in Ca2+ depletion with depth along the t-tubule. Thus Ca2+ binding within the t-tubules significantly affects variations of tubular [Ca2+] induced both by changes in extracellular [Ca2+] and by Ca2+ transport across the tubular membrane. We conclude that such models can provide detailed information that is neglected if the t-system is described by lumped models. This may be important because ion transport proteins may be non-uniformly distributed along the t-tubule (Scriven et al. 2000).
University of Oxford (2004) J Physiol 561P, PC5
Communications: MODELLING THE DISTRIBUTION OF [Ca2+] WITHIN THE CARDIAC T-TUBULE – EFFECTS OF Ca2+ CURRENT DISTRIBUTION AND CHANGES IN EXTRACELLULAR [Ca2+]
Simurda,Jiri ; Pasek,Michal ; Christe,Georges ; Simurdova,Milena ;
1. Department of Physiology, Faculty of Medicine, Masaryk University, 662 43, Brno, Czech Republic. 2. Institute of Thermomechanics, Czech Academy of Science - branch Brno, 616 69, Brno, Czech Republic. 3. INSERM E0219, DRDC/DVE, CENG, F-32054, Grenoble, France.
View other abstracts by:
Figure 1: Depletion of Ca2+ in the t-tubule lumen induced by activation of ICa
Where applicable, experiments conform with Society ethical requirements.