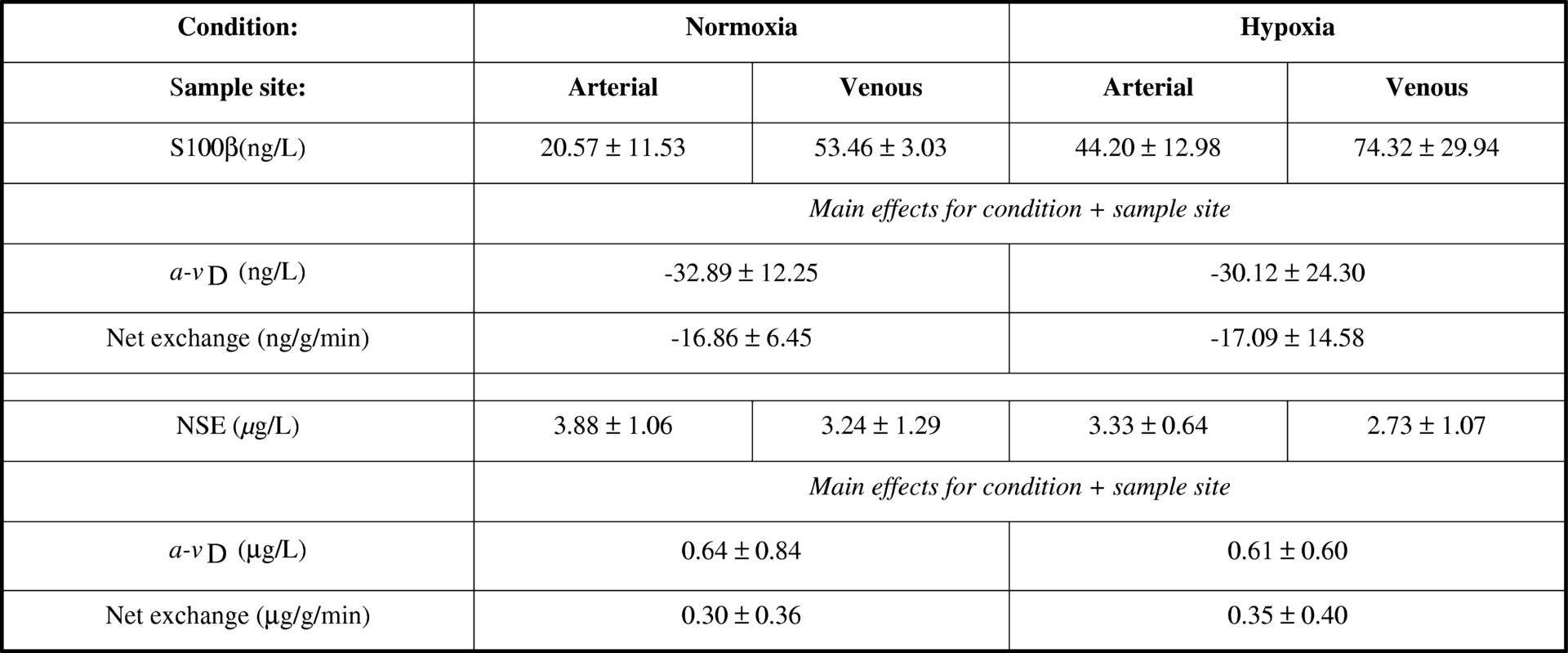
It has been suggested that inspiratory hypoxia may cause molecular opening of the blood brain barrier (BBB) in the absence of structural neuronal-parenchymal damage subsequent to a systemic accumulation of free radicals (Bailey et al., 2009). Therefore the current study aimed to establish changes in regional metabolism, specifically the trans-cerebral exchange of S100β and neuron-specific enolase (NSE) to distinguish potential “barrier disruption” from structural “brain tissue damage” caused by hypoxia. Ten healthy males aged 27 (mean) ± 4 (SD) years were examined in normoxia and following 9h passive hypoxia (12.9%O2). Internal jugular venous and radial arterial bloods were collected simultaneously for the measurement of serum S100β and NSE by ELISA. Global cerebral blood flow (CBF) was measured using the Kety-Schmidt technique (Kety & Schmidt, 1945) and global cerebral plasma flow (CPF) determined as CBF x (1-haematocrit). Trans-cerebral net exchange was calculated as the arterio-jugular venous concentration difference x CPF. Data were analysed using a two-way repeated measures ANOVA and post-hoc Bonferroni-corrected paired samples t-tests. Hypoxia was associated with a general increase in S100β (arterial inflow and venous output) whereas NSE decreased (Table 1). The net cerebral output of S100β and uptake of NSE observed during normoxia was shown to persist without any further changes being incurred during hypoxia. These findings provide the first regional molecular evidence to suggest that hypoxia causes subtle barrier disruption in the absence of neuronal-parenchymal brain injury.
University College Dublin (2009) Proc Physiol Soc 15, PC200
Poster Communications: Molecular evidence for blood-brain barrier disruption in the absence of structural tissue damage during acute exposure to inspiratory hypoxia
K. A. Evans1, S. Taudorf2, R. M. Berg2, C. Lundby3, J. McEneny4, I. S. Young4, P. E. James5, J. M. McCord6, B. K. Pedersen2, K. Moller2,7, D. M. Bailey1
1. Neurovascular Research Laboratory, University of Glamorgan, Pontypridd, United Kingdom. 2. Centre of Inflammation & Metabolism, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark. 3. Copenhagen Muscle Research Centre, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark. 4. Centre for Public Health, Queen's University, Belfast, United Kingdom. 5. Wales Heart Research Institute, School of Medicine, Cardiff University, Cardiff, United Kingdom. 6. Division of Pulmonary Sciences & Critical Care Medicine, University of Colorado, Denver, Colorado, USA. 7. Departments of Cardiothoracic Anaesthesia & Intensive Care Unit 4131, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
View other abstracts by:
Table 1 Brain-specific proteins<#13> Values are mean &#177; SD; NSE; a-vD, arterial minus venous concentration difference; main effect for condition indicates a pooled (arterial + venous) difference (P &lt; 0.05) between normoxia vs. hypoxia; main effect for sample site indicates a pooled (normoxia + hypoxia) difference (P &lt; 0.05) between arterial vs. venous.
Where applicable, experiments conform with Society ethical requirements.