Plasma phosphate levels are maintained by the type II sodium phosphate cotransporter family. NaPi-IIb mediates phosphate uptake from the small intestine followed by selective reabsorption from the renal proximal tubule by NaPi-IIa. It has previously been shown that expression of these cotransporters in MDCK cells results in differential sorting patterns. Mouse NaPi-IIa (mIIa) is expressed in both apical and basolateral membranes, whereas NaPi-IIb isoforms from flounder (fIIb) and mouse (mIIb) are preferentially sorted to the apical membrane (Hernando et al. 2000).
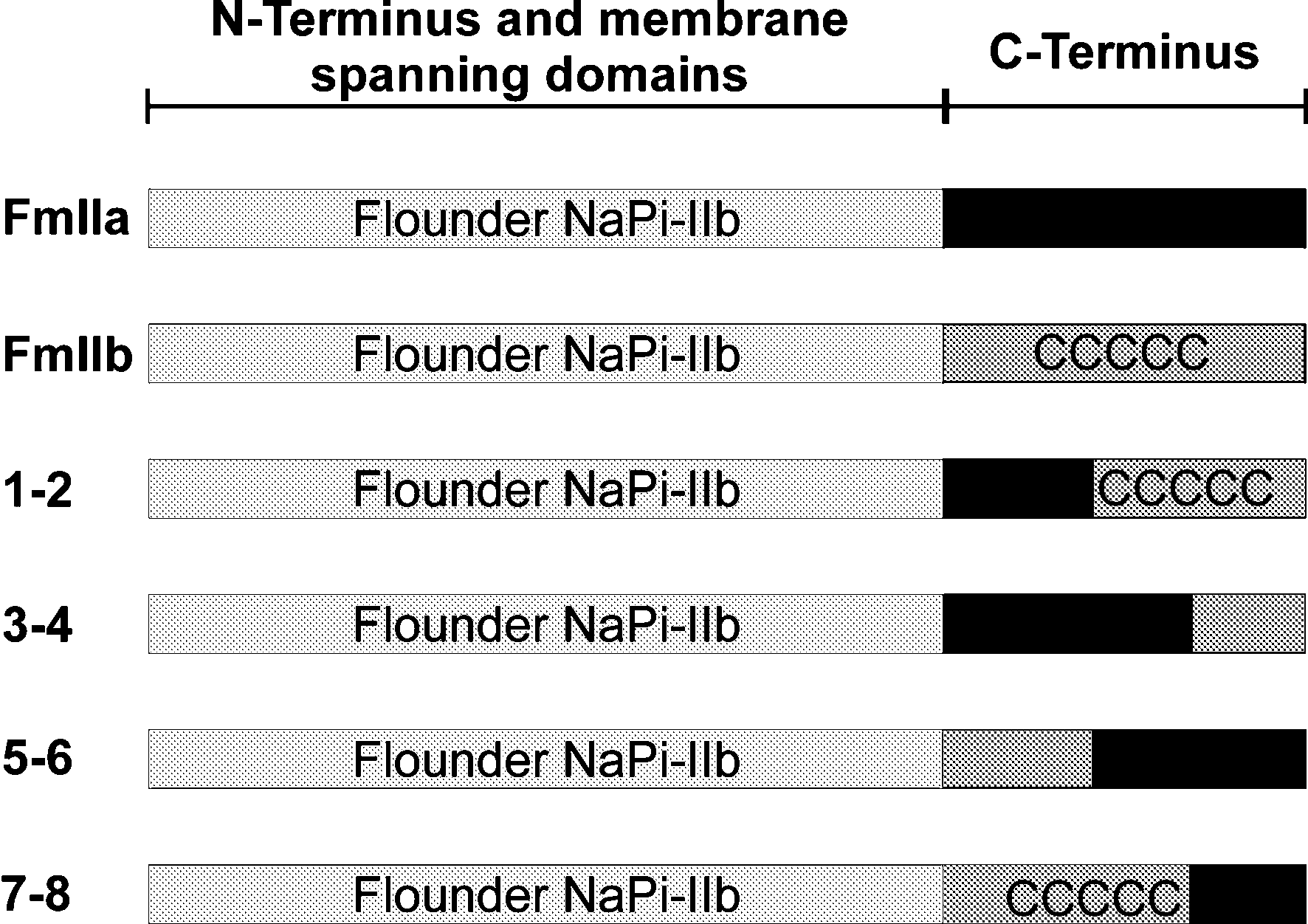
A ClustalW alignment of mIIa, mIIb and fIIb highlighted that the major differences between the type IIa and type IIb isoforms lie in the N- and C-termini as well as in the large extracellular loop (ECL3). We identified the C-terminus as the most likely candidiate for containing sorting information and so generated chimeras of these isoforms which allowed the C-termini to be switched. A schematic diagram of the generated chimeras used in this study is shown in Fig. 1.
MDCK cells grown on glass coverslips were transfected with the chimeras (Qiagen effectene), grown to confluency and fixed with 3 % paraformaldehyde. All chimeras contained an N-terminal EGFP tag, which allowed the recombinant proteins to be localised by confocal laser scanning microscopy.
As expected, exchanging the fIIb C-terminus with the same region from mIIb (chimera FmIIb) had no effect on the localisation. However, exchanging the C-terminus for that of mIIa (chimera FmIIa) led to a shift in the expression pattern to both apical and basolateral domains. Therefore, we concluded that the sorting signal lies within the C-terminus.
The C-termini of NaPi-IIb isoforms contain a stretch of cysteine residues that are absent in NaPi-IIa sequences. We investigated the role of this region in protein sorting by making four new chimeras of flounder NaPi-IIb with the C-terminus consisting of a combination of mIIa and mIIb (see Fig. 1).
Chimeras containing the cysteine-rich region from mIIb (1-2 and 7-8) were targeted to the apical membrane whereas chimeras lacking this region (3-4 and 5-6) were localised both in apical and basolateral membranes. These results strongly suggest an important role of the cysteine-rich region of the NaPi-IIb C-terminus for the correct targeting of this protein.