Greater understanding of physiological mechanisms driving parturition is needed if we are to develop more effective therapies for managing preterm and post-term birth. The problem of preterm birth (defined as birth before completion of 37 weeks gestation) exemplifies why action needs to be taken; it affects ~10% of all live births worldwide, acts as the leading cause of mortality for children under 5 years old, and presents a substantial risk of serious ill-health for surviving babies throughout the lifecourse [1-2]. Preterm birth can occur either spontaneously or by clinical intervention; spontaneous preterm labour (sPTL) typically accounts for almost half of all preterm births [3]. There are currently no reliable therapies available for preventing sPTL [4] due to notable gaps in our understanding of maternal-fetal interactions involved in coordinating the onset of spontaneous labour (for both preterm and term pregnancies). There is consensus that the myometrial and decidual tissue layers of the uterus are important contributors to labour. Myometrium predominantly comprises smooth muscle, which produces the uterine contractions that are characteristic of labour. Decidua is the mucosal lining between the myometrium and placenta/fetal membranes, which is integral to maternal-fetal immunomodulation and inflammatory events during parturition.
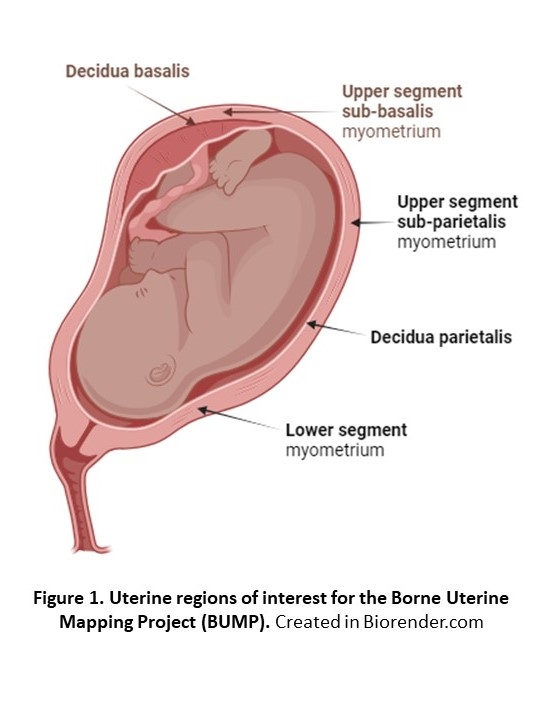
This talk presents the feasibility phase of the Borne Uterine Mapping Project (BUMP), which utilises a collaborative multidisciplinary approach to establish robust experimental protocols using innovative molecular mapping technologies, with the aim to explore multi- cellular and tissue processes that underpin the process of human labour. We applied bulk approaches for RNA-seq and proteomics to profile five distinct uterine sites (Figure 1) using biopsies acquired from term pregnancies undergoing Caesarean section while either not in labour or in early/established labour (LREC # 10/H0801/45; n=8 per group); single cell (sc) RNA-seq, single nucleus (sn) RNA-seq, spatial transcriptomics and sc proteomics were also used for biopsies from a separate cohort of not in labour and established labour Caesarean sections (n=4 per group).
Data from bulk approaches to RNA-seq and proteomics demonstrated regional differences between the five uterine sites of interest, which has important implications for understanding co-ordination of uterine contractile function. However, far less differences in gene and protein expression were significantly detected for labour status, especially for early labour. This supported the need to utilise scRNA-seq, snRNA-seq, spatial transcriptomics and sc proteomics, which delineate changes in mRNA or protein abundance between different cell types that exist in heterogenous tissues. Comparisons of scRNA-seq and snRNA-seq data for the same tissue type (decidua parietalis) will be made, and we will examine how well snRNA-seq can identify different cell types and their labour-related transcriptomic changes for three uterine regions according to our findings so far. Preliminary spatial transcriptomics data from upper segment (sub-parietalis) biopsies will also be presented to highlight the value of visualising changes in intact tissues for exploring how neighbouring cell types coordinate to mediate labour. Finally, contributions of these data to our understanding of how the timing of birth is regulated will be discussed, and we will present plans to proceed with the analysis of samples from preterm pregnancies.