Cultures of neonatal cardiac myocytes are widely used as in vitro cardiac tissue models. Fibroblasts, which constitute one of the largest cell populations in the heart, are usually considered a ‘nuisance’ in these models, even though they are important in biochemical, mechanical and, possibly, electrical signalling in situ. In rabbit sino-atrial node tissue, fibroblasts express connexin40 (Cx40) and connexin45 (Cx45), and the latter appears to be involved in fibroblast-myocyte coupling (Camelliti et al. 2002). In neonatal cell culture, myocytes and fibroblasts also form functional gap junctions, which have been attributed to Cx43 (Rook et al. 1992). A systematic study, however, of in vitro fibroblast-myocyte and fibroblast-fibroblast coupling has not yet been performed, and would require both connexin labelling and cell type identification.
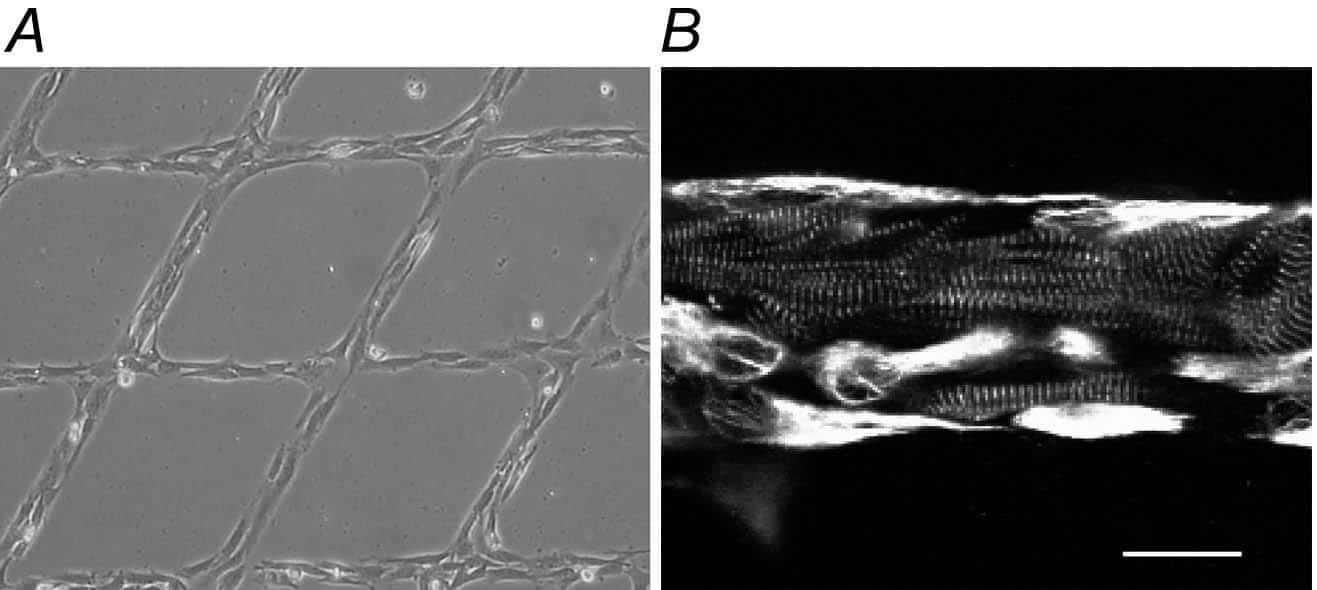
In the present study, we use a micro-fluidic technique, developed by the UCSD lab of Andrew McCulloch (Gopalan et al. 2003), to deposit a 2D criss-cross pattern of intersecting lines (28 µm wide) of extracellular matrix proteins (here type I collagen). Cell suspensions from heart of humanely killed neonatal rats containing either preferentially fibroblasts or a mixture of myocytes and fibroblasts are seeded. This gives rise to a 2D cardiac tissue model (Fig. 1A) with improved cyto-architectural properties of both myocytes and fibroblasts (Fig. 1B). Using triple immunocytochemical labelling (anti-vimentin antibodies to mark fibroblasts, anti-myomesin antibodies for myocytes, and anti-Cx40, anti-Cx43 or anti-Cx45 antibodies for gap junctions) and confocal microscopy we studied gap junction localisation relative to coupled cell types. We found sparse punctate Cx40, Cx43 and Cx45 labelling between homologous cell types, both in fibroblast cultures and fibroblast myocyte co-cultures. Cx43 and, to a lesser extent, Cx45 appeared to also be involved in heterologous fibroblast-myocyte coupling.
Thus, cardiac fibroblasts in vitro express multiple connexin isoforms and form gap junctions at the point of contact both with fibroblasts and myocytes, via Cx43 or Cx45. Taking into account the overall low density of connexin immunolabelling found in this study, we cannot rule out contributions by other connexin isoforms to cell communication in this 2D structured cardiac tissue models.
We thank Prof. Andrew McCulloch for access to micro-patterning tools and the BBSRC for financial support. PK is a Royal Society Research Fellow.