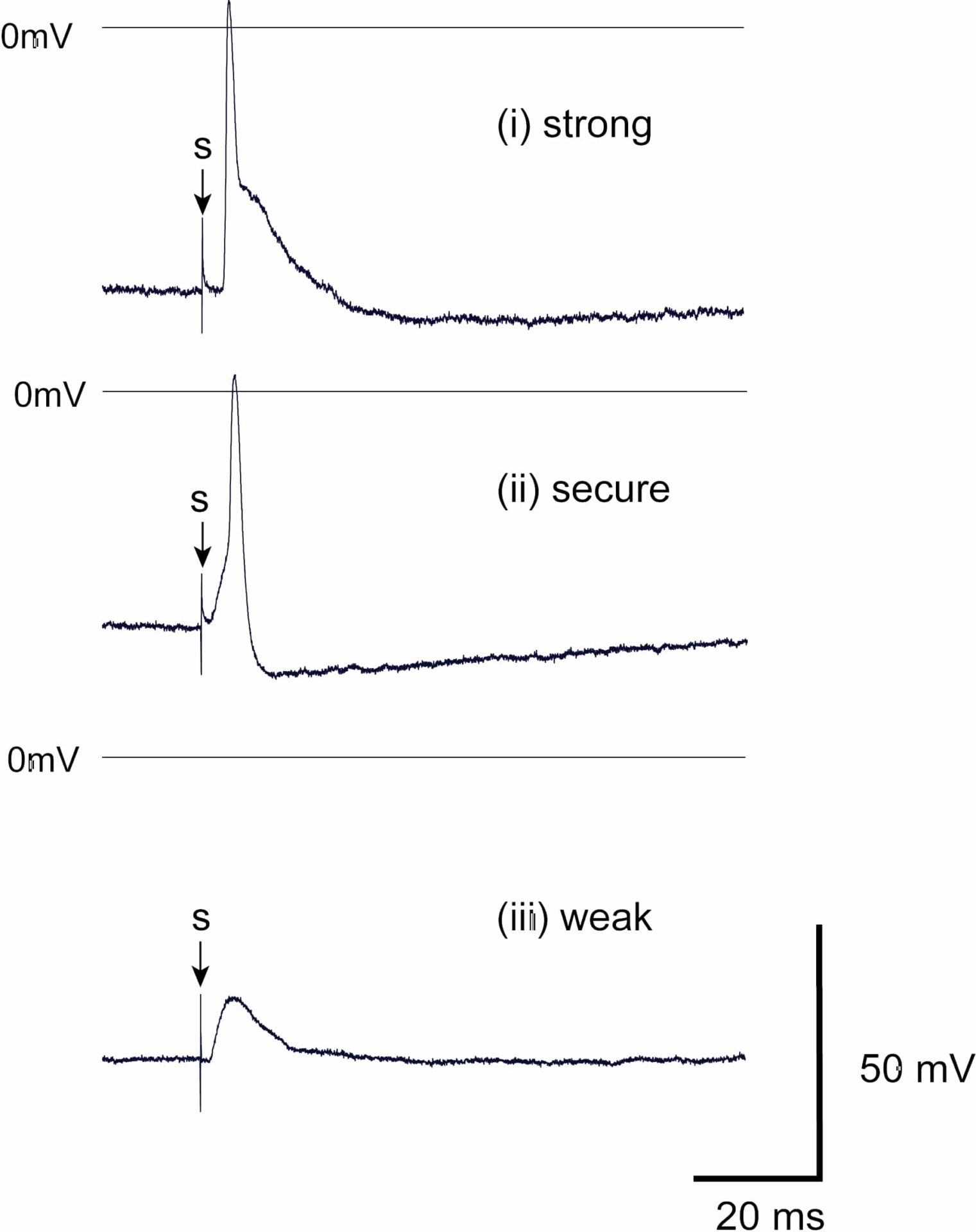
There is accruing interest in the cardiac nervous system, particularly its role in the generation of cardiac arrhythmias and sudden cardiac death (Fukuda et al., 2015). Intracardiac ganglia (ICG) form the final common pathway for the cardiac nervous system. Further understanding of how ICG neurones integrate information is likely to contribute to improved or novel therapeutic strategies for the prevention of arrhythmias. Although there are several reports characterising murine ICG neurones (e.g. Brown et al., 2018; Hoard et al., 2007), to our knowledge there are no studies on ganglionic transmission. Using intracellular microelectrode recording and nerve stimulation, we describe the intrinsic electrical characteristics and synaptic transmission in intracardiac ganglia of the adult mouse heart in situ.Young (6 week) male adult c57BL/6NJAusb mice (Australian BioResources, NSW) were terminally anaesthetized with Fluothane as approved by the University of Wollongong Animal Ethics Committee (AE16/10). The heart and lungs quickly excised and the right atrial ganglion plexus and underlying myocardium isolated from the dorsal surface of the atria. Experiments were performed at 36°C using a bicarbonate buffered physiological salt solution, and atrial contractions were suppressed using blebbistatin (5 µM, Cayman Chemical, Ann Arbor, MI USA). The mean cell capacitance of mouse ICG neurones was 26.7 ± 8.9 pF and the values for the resting membrane potential and input resistance in mouse ICG neurones were -49.2 ± 4.8 mV and 201 ± 80 MΩ (SD, n=20) respectively. Somatic action potentials (AP) were evoked by short current pulses (2 ms). The AP after-hyperpolarization amplitude and time to 50% recovery being 9.9 ± 2.6 mV and 37.8 ± 25.3 ms (n=15), respectively. Synaptic responses to single stimuli of the vagus or interganglionic nerves were divided into three groups with the following relative frequency: strong 15/20, secure 4/20 and weak 1/20 (see Figure 1). The efficacy of synaptic transmission as a function of stimulation frequency (5, 10, 20, 50 and 100 Hz) was studied in neurones with strong synaptic responses. Trains of stimuli (20), were applied and AP discharge monitored in the postganglionic neuronal soma. Synaptic efficacy was determined as the percentage of postsynaptic APs as a function of nerve stimulation. The number of successful APs, 100% at 5-50 Hz decreased to 92 ± 13% SD (n=10) at 100 Hz stimulation. This framework will now be used to organize the characterization of the ICG in specific genetically modified animals.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, PC207
Poster Communications: Neurotransmission in mouse intrinsic cardiac ganglia in situ
A. A. Harper1,2, D. J. Adams2
1. Life Sciences, University of Dundee, Dundee, United Kingdom. 2. Illawarra Health & Medical Research Institute (IHMRI), University of Wollongong, Wollongong, New South Wales, Australia.
View other abstracts by:
Figure 1. Representative synaptically-evoked postsynaptic responses recorded from mouse ICG neurones in response to vagal nerve stimulation. Postsynaptic responses were classified as:(i) strong where the AP arises early during the EPSP, (ii) secure where the AP arises late in the EPSP, when present, or without an obvious EPSP, and (iii) weak where nerve stimulation (s) evokes a subthreshold EPSP.
Where applicable, experiments conform with Society ethical requirements.