Sodium-glucose cotransporter 2 inhibitors attenuate left atrial electrical remodelling induced by high-fat diet
Rina Sha1, Olivia Baines1, Abbie Hayes1, Sophie Broadway-Stringer1, Manish Kalla1,3, Ming Lei4, Katja Gehmlich1, Andrew P Holmes1,2, Christopher O’Shea1, Davor Pavlovic1
1 Cardiovascular Sciences, University of Birmingham, UK. 2 Institute of Clinical Sciences, University of Birmingham, UK. 3 Department of Cardiology, Queen Elizabeth Hospital, UK. 4 Department of Pharmacology, University of Oxford, UK.
Introduction:
Obesity significantly increases the risk of developing atrial arrhythmias. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, originally used for diabetes management, have shown potential anti-arrhythmic benefits, though their direct effects on atrial cardiac electrophysiology in the setting of obesity are unclear. Our work aims to define the atrial proarrhythmic alterations induced by high-fat diet (HFD) and to test if early treatment with SGLT2 inhibitors can attenuate observed changes.
Methods:
CD1 mice (5–8 weeks old) were randomised into three groups: normal chow, HFD, and HFD + SGLT2 inhibitor. The normal chow group received standard chow and water for 20 weeks. The HFD group were fed HFD and high-fructose (15%) water for 20 weeks. During the last
six weeks, the HFD + SGLT2 inhibitor group received dapagliflozin (3 g/kg/day) in their drinking water. ECGs and echocardiography were performed at weeks 14 and 20. At 20 weeks, blood and urine samples were collected to measure glucose levels. An Olink® Target 48 proteomic cytokine panel was used to assess changes in protein expression of 45 key cytokines in plasma. Left atrial electrical activity was assessed using cardiac optical mapping, measuring action potential duration (APD) and conduction velocity (CV). Effective refractory
periods (ERP) were assessed using an S1-S2 pacing protocol. Optical mapping data analysis was performed with ElectroMap.
Results:
After 20 weeks, the HFD and HFD+SGLT2 inhibitor groups showed significantly greater body weight gain than the normal chow group (28.32 ± 2.66, 25.89 ± 3.45, and 9.43 ± 0.93 g, respectively; p < 0.0001). As expected, the HFD + SGLT2 inhibitor group showed glucose in
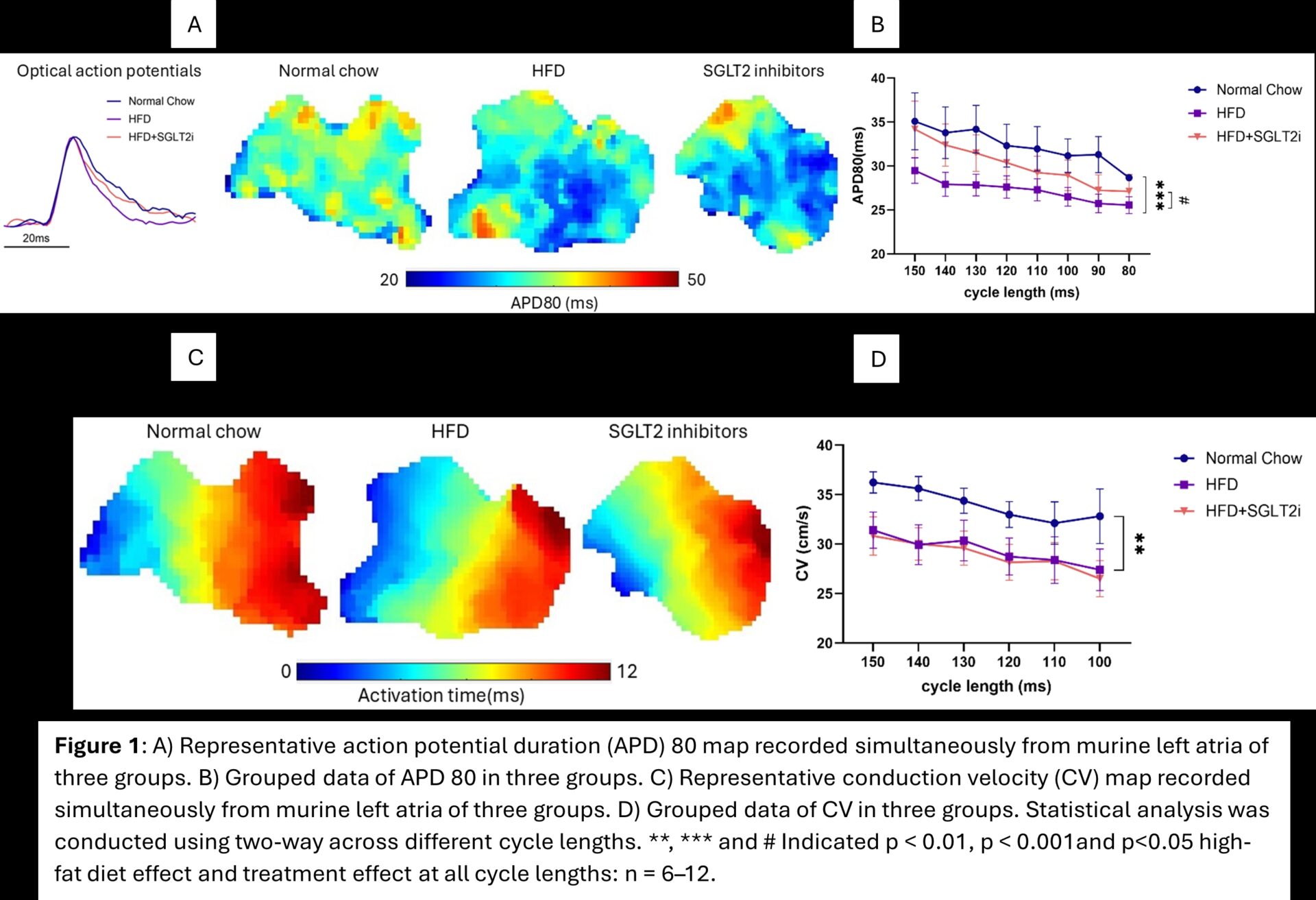
their urine. No differences were observed in left atrial weight, blood glucose, cytokine biomarkers or ECG parameters. Interestingly, the SGLT2 inhibitor group showed increased left ventricular wall thickness (Anterior: 1.413 ± 0.0793, 1.525 ± 0.0539, 1.600 ± 0.0781mm, respectively. p>0.05. Posterior: 1.624 ± 0.0861, 1.683 ± 0.0757, 1.952 ± 0.0929mm, respectively. p<0.05). HFD induced substantive pro-arrhythmic changes in the left atria. HFD shortened APD at 30%, 50% and 80% repolarisation (APD30, APD50, APD80), decreased ERP and slowed left atrial CV, compared to the normal chow group. SGLT2 inhibitors attenuated the repolarisation deficits, by increasing APD30, APD50 and APD80 while reducing APD heterogeneities and attenuated the HFD-induced ERP changes (57.60 ± 3.92, 42.40 ± 3.12, 47.40 ± 3.41ms, respectively; p > 0.05).
Conclusions:
HFD induces pro-arrhythmic changes in murine left atria. Interestingly, this occurs in the absence of changes in systemic cytokine/inflammatory markers. SGLT2 inhibitors exhibited substantive anti-arrhythmic effects by modulating cardiac electrophysiology. These findings suggest that early treatment with SGLT2 inhibitors may prevent obesity-induced atrial electrical remodelling. Further studies in obese animal models and patients are warranted to explore their therapeutic role in arrhythmia prevention.