Gradual loss of sarcoplasmic reticular (SR) calcium during a rest period is responsible for the rest-induced decay of force of contraction in the mammalian myocardium (Reiter, 1988). The effect of two organic calcium channel blockers (OCCB), verapamil and diltiazem, on a similar ‘rest-induced decay’ (RID) in the frog myocardium suggests a new mechanism of action of these drugs.
Strips of frog ventricle from isolated hearts of pithed frogs (Rana hexadactyla) were mounted in a temperature-controlled bath (25Ð28 °C) continuously perfused with a well-oxygenated solution of the following composition (mM): NaCl 110, KCl 2, CaCl2 2, MgCl2 1, Hepes 10 and glucose 10; pH 7.4. The ventricular strip was subjected to field stimulation with silver electrodes at 0.2 Hz frequency and the force of contraction recorded with a force-transducer and chart recorder. The regular rhythm was interrupted by varying rest-periods ranging from 10 to 180 s.
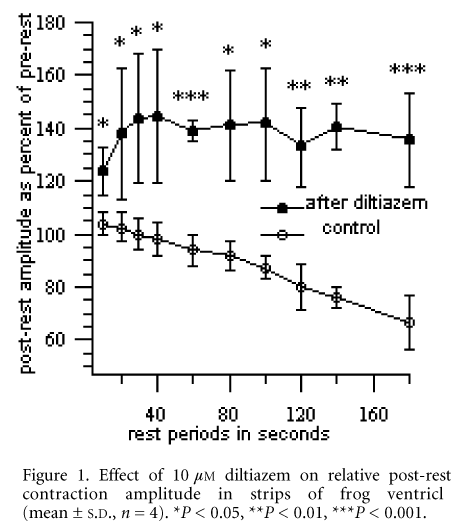
The amplitudes of the contractions immediately before and after each rest period were compared. In general, the amplitude of the post-rest beat was less than that of the pre-rest beat. Such ‘rest-induced decay’ was greater with longer rest periods. The RID was statistically significant (P < 0.05 with paired t tests) for rest periods more than 100 s.
Diltiazem (10 µM), an OCCB, changed the pattern of ‘RID’ in the control solution to a ‘rest-induced potentiation’ (RIP) in the same preparation. The drug was nevertheless negatively inotropic, but the relative post-rest amplitude was significantly enhanced at all rest periods compared with control solution (Fig. 1, P < 0.05 with paired t tests). A similar significant RIP was seen with verapamil (10 µM) but not with nifedipine (10 µM), also an OCCB.
The only known mechanism of action of OCCBs, used in the treatment of hypertension and cardiac arrhythmias is sarcolemmal calcium channel blockade, like the inorganic substance cadmium chloride. The reported RIP with verapamil and diltiazem cannot be explained by their known mechanism of action, because cadmium chloride (10 µM), though negatively inotropic, did not alter the RID of force seen in control solution.
In conclusion, the RID of force seen in frog myocardium is not due to a decreased calcium influx via the sarcolemmal calcium channels, because it is not abolished by cadmium blockade of these channels. It is therefore related to a decrease in SR calcium output following a rest period, which can be explained by a diastolic SR calcium leak as in the mammalian myocardium. We propose that the RIP in the presence of verapamil and diltiazem is due to blockade of such a leak.
S. Subramani thanks The Wellcome Trust for the travel grant which made this presentation possible.
All procedures accord with current national and local guidelines.