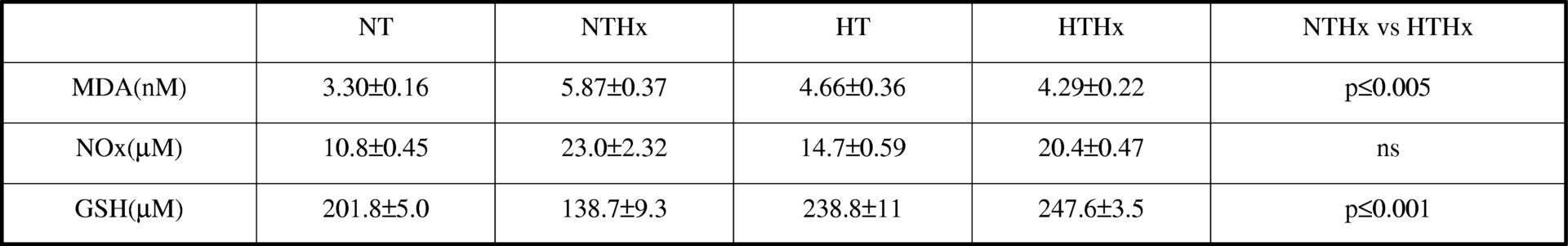
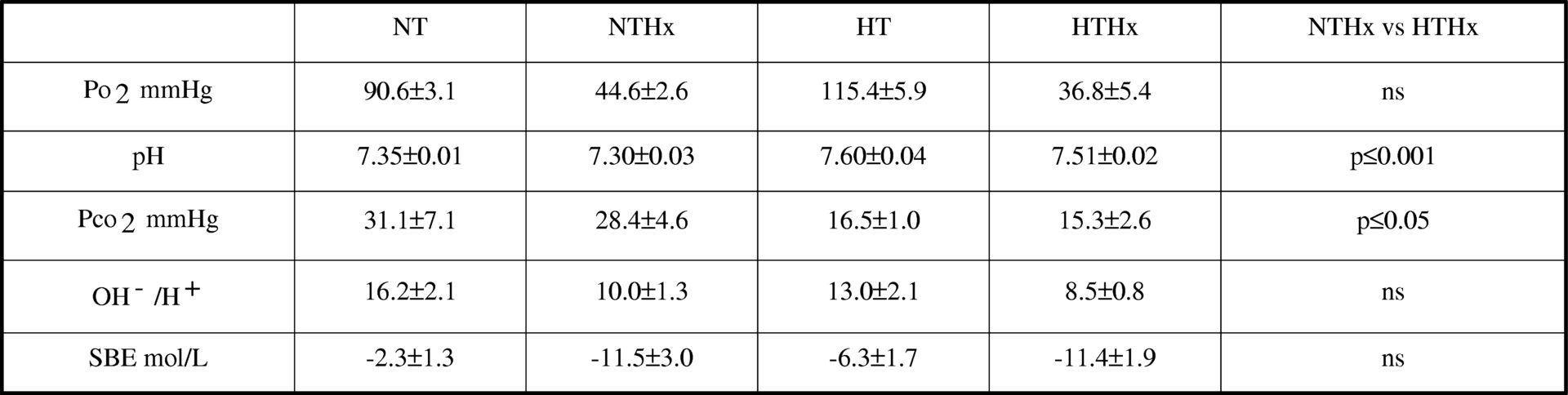
The use of deep hypothermia as a protective factor in hypoxia has been controversial. (Matthew et al., 2002; Riess et al., 2004). The purpose of this study was to describe the blood acid-base parameters and the profile related to oxidative stress, malondialdehyde, nitric oxide and glutathione in plasma, in deep hypothermic Sprague Dawley rats (21.5°C)breathing room air or hypoxic air (10% O2 in N2 ), compared with normothemic animals also breathing room air or hypoxic air. Rats were anaesthetized I.P. (intraperitoneally) with sodium pentobarbitone (60mg/Kg b.w.) and maintained with respiratory aid. The animals were humanely killed with an I.P. overdose of anaesthetic. Data were analyzed by the two-way ANOVA using the Student-Newman-Keuls test to identify significant differences (p≤0.05). Hypoxia exposure results in an oxidative stress with an increase in malondialdehyde, nitric oxide and a decrease in glutathione; however, if hypothermia is previously applied the situation is reversed with a stabilization of the malondialdehyde and an increase in the antioxidant glutathione (Table 1). On the other hand, the determination of pH, Pco2 and the ratio [OH–/H+] discarded a respiratory imbalance but showed a mild metabolic acidosis in the hypothermic groups (Table 2). We proposed metabolic acidosis as a mechanism which explains this protection since it helps to keep the intracellular reducing power, preserving glutathione and avoiding intracellular alkalinisation.
University College Dublin (2009) Proc Physiol Soc 15, PC59
Poster Communications: Oxidant/antioxidant status in hypoxic rats after submission to deep hypothermia
T. Carbonell1, N. Alva1, J. Palomeque1
1. Physiology, University of Barcelona, Barcelona, Spain.
View other abstracts by:
Table 1. Plasma oxidative stress-indicators<#13> Values are the mean &#177; SEM
Table 2. Acid base parameters<#13>
Where applicable, experiments conform with Society ethical requirements.