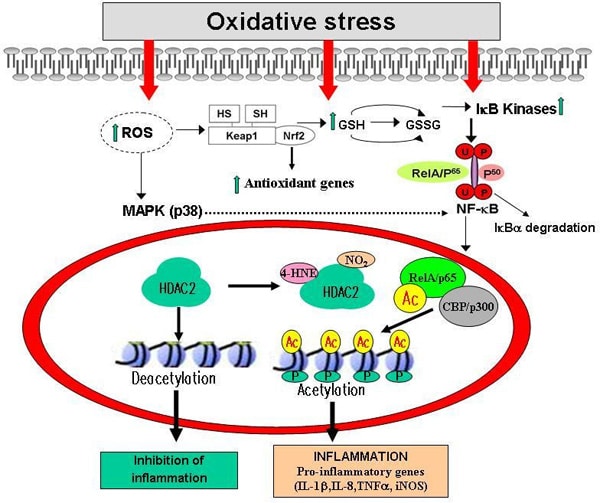
Oxidative stress and inflammation are major hallmarks of various chronic inflammatory diseases and cancer. We determined the role of oxidative stress in triggering the inflammatory response in the lungs in response to cigarette smoke by assessing the role of NADPH oxidase, and redox sensitive transcription factors Nrf2 and NF-κB in pro-inflammatory cytokine release. 1) Role of NADPH oxidase: NADPH oxidase is a multisubunit complex that generates superoxide anion in one-electron reduction of molecular oxygen using electrons supplied by NADPH. We determined the effect of targeted ablation of components of NADPH oxidase (p47phox-/- and gp91phox-/-) on inflammatory response in lungs against detrimental effects of cigarette smoke (CS). Surprisingly, CS exposure caused significant influx of neutrophils in bronchoalveolar lavage fluid (BALF) and macrophages in lung tissue of wild-type mice, which were augmented in p47phox-/- and gp91phox-/- mice compared to respective controls. Lung levels of NF-κB, and pro-inflammatory cytokines, such as KC, IL-6, MCP-1 and TNF-α were significantly increased in wild-type mice and were augmented after CS exposure in both the knockout mice. These data suggest that genetic ablation of p47phox-/- and gp91phox-/- components of NADPH oxidase assembly decreases oxidative stress but enhances susceptibility to proinflammatory effects of cigarette smoke. 2) Role of Nrf2: Nuclear erythroid related factor 2 (Nrf2) is a redox sensitive transcription factor that is involved in transcriptional regulation of many antioxidant genes. We determined the role of Nrf2 in cigarette smoke-mediated regulation of antioxidant genes in macrophages and lung epithelial cells. Treatment of human macrophages and alveolar epithelial cells to a variety of oxidants decreased Nrf2 nuclear translocation by its post-translational modification which was associated with sequestration of Nrf2 in the cytoplasm. Immunohistochemistry data on human peripheral lung tissue showed sequestration of Nrf2 predominantly in the cytoplasm of airway epithelium, alveolar type II cells and macrophages in smokers and patients with COPD compared with non-smokers. Cytoplasmic sequestration of Nrf2 was associated with increased NF-κB translocation in the nucleus. These data suggest that cigarette smoke extract caused sequestration of Nrf2 in the cytoplasm by its post-translational modifications associated with activation of NF-κB in macrophages and epithelial cells as well as in human lungs of smokers and COPD. 3) Role of NF-κB and histone acetylation: Reactive oxygen species (ROS) play a key role in enhancing the inflammation through the activation NF-κB and alteration in nuclear histone acetylation and deacetylation (chromatin remodeling) leading to sustained gene expression of pro-inflammatory mediators in the lung (Figure). Histone acetylation is reversible and is regulated by a group of acetyltransferases (HATs) which promote acetylation, and deacetylases (HDACs) which promote deacetylation. We show that oxidative stress induced by cigarette smoke enhances lung inflammation through the activation of intrinsic HAT activity of co-activator molecules, and increased histone 3 phospho-acetylation leading to increased NF-κB activation. Oxidative stress also inhibits the activity of HDACs, activates cells for NF-κB transactivation and enhances inflammatory gene expression which leads to chronic inflammatory response both in monocytes and epithelial cells in vitro and in vivo in rodent lungs exposed to cigarette smoke. Increased histone acetylation was associated increased activation of IκB kinase-α (IKKα), and interaction of NF-κB with CBP leading to increased acetylation of RelA/p65 subunit of NF-κB. Down-modulation of HDAC2 was related to increased phosphorylation of HDAC2 at serine residue. These data provide new information on oxidant-mediated regulation of inflammatory response by chromatin remodeling at molecular level. Overall, we conclude that oxidants play an important role in triggering the inflammatory response either by activation of NF-κB and/or down-modulation of Nrf2. However, genetic ablation of components of NADPH oxidase assembly enhanced susceptibility to lung inflammation implicating a protective role of endogenous ROS against inflammation.
Life Sciences 2007 (2007) Proc Life Sciences, SA206
Research Symposium: Oxidative stress and redox regulation of lung inflammation
I. Rahman1
1. University of Rochester Medical Center, Rochester, NY, USA.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.