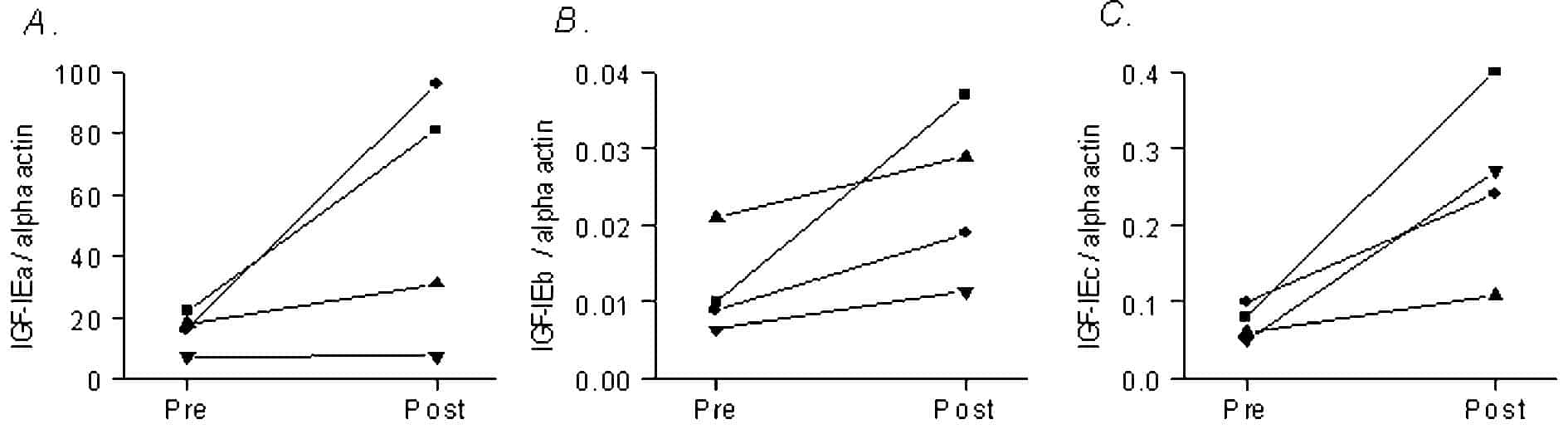
Spinal cord injury and subsequent paralysis results in a loss of muscle mass, an infiltration of connective tissue and a shift towards fast myosin isoforms in the affected muscles. In contrast to disuse, muscle will adapt to increased use and overload through mechanisms regulated by locally expressed growth factors. In this regard three transcripts of the IGF-I gene have been shown to be expressed and upregulated in young and aged human skeletal muscle (Hameed et al. 2003a,b); IGF-IEa, IGF-IEb and IGF-IEc (also known as MGF). In the present study, undertaken with local ethics committee approval, we have investigated whether paralysed tibialis anterior muscle firstly, expresses these growth factors and secondly, if so, to determine whether they remain sensitive to a severe exercise challenge imposed by chronic electrical stimulation training.
Four spinal cord injured men (mean age 39 ± 9 S.D. years ) completed 4 weeks of low frequency electrical stimulation training of the tibialis anterior muscle (2-6 h per day; at 10 Hz, 5 s on 5 s off under isometric loading conditions, see Harridge et al. 2002). Following local anaesthesia with 1 % lidocaine, biopsies of the tibialis anterior muscle were taken before and following 4 weeks of training. The mRNA levels of the three IGF-I splice variants were determined using real time quantitative PCR (Hameed et al. 2003a). Measurement of α actin mRNA levels showed no significant change as a result of the electrical stimulation training(13.1 ± 6.5 v 10.2 ± 7.0 ng mRNA 10-7 / µg RNA). The IGF-I mRNA data were normalised to these values to remove variability caused by connective tissue RNA contributing to total RNA.
All isoforms were expressed in the paralysed muscle and increased as a result of the electrical stimulation training protocol in each individual (Fig. 1). Although, statistically this only reached significance for MGF (P < 0.05, paired t test).
The data show that paralysed muscles can increase the expression of all three isoforms of IGF-I as a result of an exercise challenge. This is encouraging as it is becoming clear that the isoforms have important, but differing physiological roles both in muscle (such as stimulating protein synthesis and triggering the activation of satellite cells) and nerve repair.
Work was supported by grants from the Danish Medical Research Council (no. 9802636, The Danish Research Council (no. 504-14)and the Wellcome Trust.