University of Leeds (2002) J Physiol 544P,
S298
Research Symposium: Physical principles of mechanosensitive channel gating by bilayer deformation forces
Boris Martinac* and Eduardo Perozo†
*Department of Pharmacology, Queen Elizabeth II Medical Center, University of Western Australia, Crawley, WA 6009, Australia and †Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22906, USA
View other abstracts by:
Mechanosensitive (MS) ion channels have been documented in cells belonging to a wide variety of prokaryotic and eukaryotic organisms (Hamill & Martinac, 2001). The channels show great diversity in their conductance, selectivity and voltage dependence, while sharing the property of being gated by mechanical force exerted on cell membranes. They act as membrane-embedded mechanoelectrical switches, which open in response to lipid bilayer deformations. This process is critical to the response of living organisms to direct physical stimulation, as in touch, hearing, osmoregulation and other physiological responses. In prokaryotes MS channels were first documented in Bacteria and later in Archaea. Among prokaryotic MS channels studied to date, the best characterized are the MS channels of E. coli, which has three types of MS channels based on their conductance and sensitivity to applied pressure: MscM (M for mini), MscS (S for small) and MscL (L for large). Bacterial MS channels were the first shown to sense directly membrane tension in the lipid bilayer caused by external mechanical force applied to the cell membrane. The validity of the bilayer model has been well documented for MS channels in bacteria as well as in archaea (Hamill & Martinac, 2001).
MscL, the bacterial MS channel of large conductance, has become a prototype MS channel to study the structure-function relationship in this class of ion channels. Within a few years of the cloning of the mscL gene the 3D structure of the MscL protein was determined by X-ray crystallography (Chang et al. 1998), showing that the MscL channel is a homopentamer. A site-directed spin labelling (SDSL) study of MscL using electronparamagnetic resonance (EPR) spectroscopy provided complementary structural information about the conformation of the closed MscL channel (Perozo et al. 2001). To probe the molecular mechanism of how mechanical force gates MscL we have recently evaluated two physical mechanisms as triggers of MscL gating by bilayer deformation forces: (i) the energetic cost of protein-bilayer hydrophobic mismatches and (ii) the geometric consequences of bilayer intrinsic curvature.
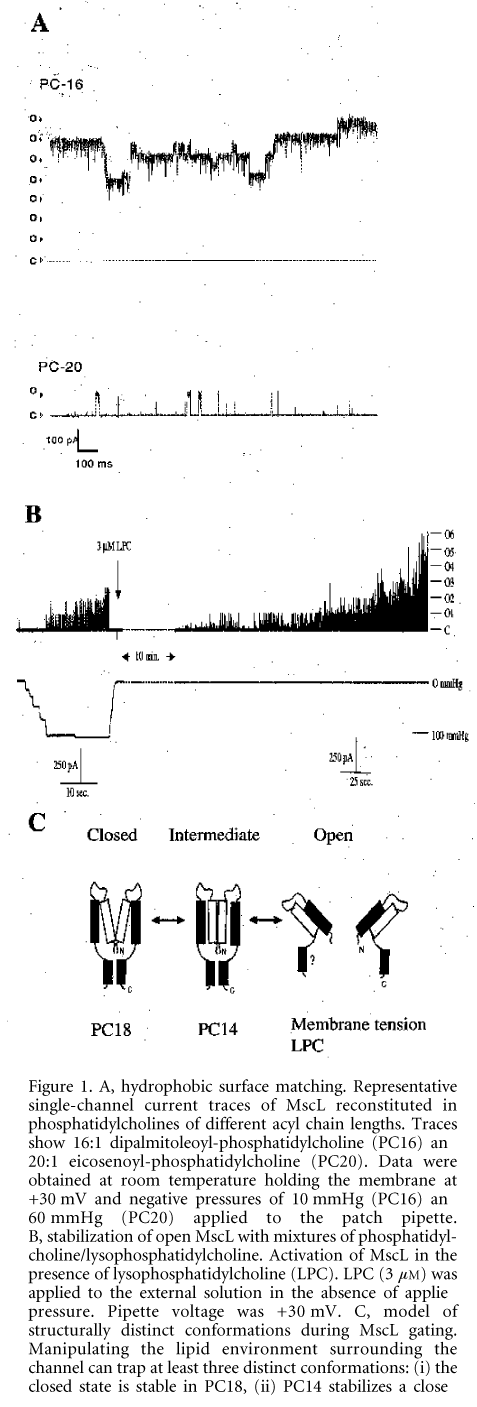
Structural changes in MscL from E. coli were studied under zero transbilayer pressures using both the patch clamp and EPR spectroscopic approaches (Perozo et al. 2002a, b). To examine the role of hydrophobic mismatch in MscL gating we recorded the activity of MscL in phosphatidylcholine bilayers of different thickness ranging from 16 (PC16) to 20 (PC20) hydrocarbons per acyl chain. We found that decreasing bilayer thickness lowered MscL activation energy (Fig. 1A). Furthermore, the EPR spectroscopic analysis indicated that a structurally distinct closed channel intermediate was stabilized in PC14 bilayers (Perozo et al. 2002b). Although hydrophobic mismatch alone was unable to open the channel, the importance of hydrophobic mismatch for MscL mechanosensitivity results from stretch-induced bilayer thinning, which stabilizes the open conformation of MscL due to a better hydrophobic match with the open compared with the closed conformation of the channel (Perozo et al. 2002a).
This work was supported in part by NIH grant R01-GM636170 (E.P.) and the McKnight endowment fund for neuroscience (E.P.), the Australian Research Council grants A00000819 and IP97054 (B.M.), and the Australian Academy of Science (Scientific Visit Award to B.M.).
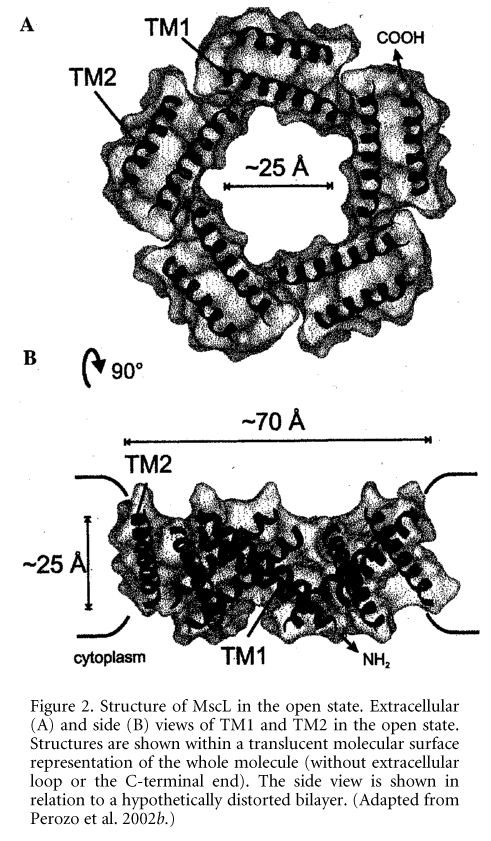
Figure 1. A, hydrophobic surface matching. Representative single-channel current traces of MscL reconstituted in phosphatidylcholines of different acyl chain lengths. Traces show 16:1 dipalmitoleoyl-phosphatidylcholine (PC16) and 20:1 eicosenoyl-phosphatidylcholine (PC20). Data were obtained at room temperature holding the membrane at +30 mV and negative pressures of 10 mmHg (PC16) and 60 mmHg (PC20) applied to the patch pipette. B, stabilization of open MscL with mixtures of phosphatidyl-choline/lysophosphatidylcholine. Activation of MscL in the presence of lysophosphatidylcholine (LPC). LPC (3 mM) was applied to the external solution in the absence of applied pressure. Pipette voltage was +30 mV. C, model of structurally distinct conformations during MscL gating. Manipulating the lipid environment surrounding the channel can trap at least three distinct conformations: (i) the closed state is stable in PC18, (ii) PC14 stabilizes a closed conformation further along the kinetic path, and (iii) the fully open state can be stabilized by addition of LPC in one leaflet of the lipid bilayer. (Adapted from Perozo et al. 2002a.)Changes in membrane intrinsic curvature induced by the external addition of lysophosphatidylcholine (LPC) produced a dramatic increase in MscL single-channel activity in the absence of applied pressure (Fig. 1B). In EPR experiments addition of LPC to the external leaflet of the liposome bilayer generated massive spectroscopic changes in the narrow constriction that forms the channel 'gate', trapping the channel in the fully open state (Perozo et al. 2002b) (Fig. 1C), which suggested that it was the asymmetry in the lateral pressure profile between the two monolayers that caused the channel to open. Using EPR spectroscopy and site-directed spin labelling we have determined the structural rearrangements that underlie MscL closed-to-open transitions (Perozo et al. 2002b). Transitions to the open state were accompanied by massive rearrangements in both TM1 and TM2 helices, as shown by large increases in probe dynamics, solvent accessibility and the elimination of all inter-subunit spin-spin interactions. The open state is highly dynamic, supporting a water-filled pore of at least 25 ü, lined mostly by the TM1 helix (Fig. 2). This pore size is in agreement with the single-channel permeation studies, which by using large organic cations indicated a MscL pore of 30-40 ü in diameter (Hamill & Martinac, 2001). Our studies suggest a plausible molecular mechanism of gating in mechanosensitive channels.\"
Figure 2. Structure of MscL in the open state. Extracellular (A) and side (B) views of TM1 and TM2 in the open state. Structures are shown within a translucent molecular surface representation of the whole molecule (without extracellular loop or the C-terminal end). The side view is shown in relation to a hypothetically distorted bilayer. (Adapted from Perozo et al. 2002b.)
Where applicable, experiments conform with Society ethical requirements.