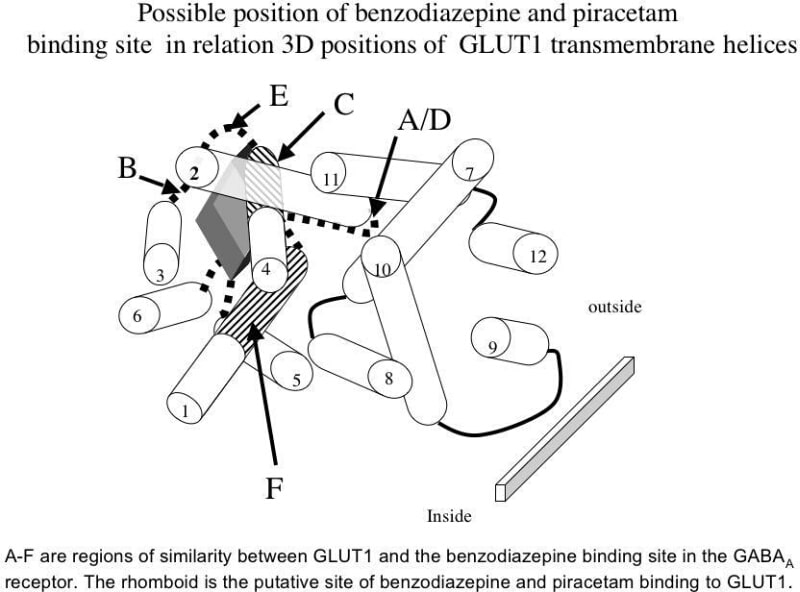
Nootropic drugs, e.g piracetam have memory enhancing properties (Moyersoons et al, 1974). They increase glucose uptake into barbiturate and benzodiazepine anaesthetised brain (Heiss et al, 1988). Barbiturates and benzodiazepines inhibit glucose transport both into brain in vivo and in vitro cell systems e.g. human erythrocytes (Haspel et al, 1999). We decided therefore to determine if piracetam and a neuropeptide analogue thyrotrophin releasing hormone (TRH), had antagonist effects on barbiturate and benzodiazapine-dependent inhibitions of glucose transport in human erythrocytes. Cells were loaded with 100mM glucose and exit rates of glucose from cells were monitored photometrically in the presence and absence of the drugs under test, using a fluorescence spectrometer as previously described (Naftalin et al, 2004). Pentobarbital and diazepam are mixed inhibitors of glucose transport, reducing both the Vmax of glucose transport and also the affinity of glucose for GLUT1. Piracetam and TRH competitively antagonise both pentobarbital or diazepam inhibition of the maximal rate of glucose exit (zero trans net exit) from erythrocytes at 25°C (Ki of Piracetam = 1.29 ± 0.17 mM, n=3; Ki of TRH = 132 ± 27.8 μM, n=3) and decrease in glucose affinity, as measured by the infinite-cis Km ( Sen-Widdas Km). A strong correlation was obtained between the observed potency of in vivo nootropic action of several nootropic drugs in rats and their in vitro Kis against pentobarbital inhibition of glucose transport (n = 9, P ≤ 0.001). Since the crystal structure of the benzodiazepine binding site to GABA A receptor is known (Sigel, 2002) we looked for similarities between amino-acid sequences in human GLUT1 and the benzodiazepine binding domains of human GABAA receptor. These homologies cluster in the N-terminal half of GLUT1. When mapped on a 3D template of GLUT1, these homologies suggest that a possible site of diazepam and piracetam interaction is a pocket outside the central hydrophilic pore region well suited to act as a non-catalytic site of inhibition (Naftalin et al, 2004).
King's College London (2005) J Physiol 565P, C2
Communications: Piracetam and TRH Antagonise Inhibition of D-Glucose transport in human erythrocytes by Barbiturates and Diazepam
Afzal, Iram ; Naftalin, Richard J; Cunnigham, Philip ;
1. Cardiovascular Biology and Medicine, Kings College London, London, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.