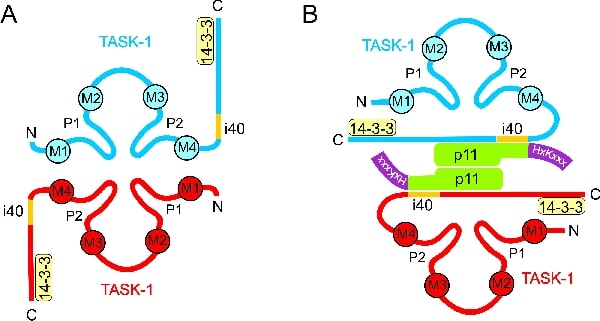
The two-pore-domain acid-sensitive potassium channels TASK-1 and TASK-3 play an important role in the central nervous system, in the heart, in smooth muscle and in many other tissues. Their surface expression is regulated not only at the transcriptional level but also at the posttranslational level. Both TASK-1 and TASK-3 have putative ER-retention signals near their N-terminal and C-terminal ends (1,2), but the functional relevance of these motifs is not yet clear. In addition, we have recently detected an ER export signal in the C-terminus of TASK-3 that promotes surface expression. There are two important accessory proteins involved in the trafficking of TASK-1: 14-3-3 proteins (2,3), which also interact with TASK-3, and p11 (3,4), which is also denoted S100A10. 14-3-3 proteins bind to the C-terminal end of TASK-1, the essential 14-3-3 binding motif is RRx(S/T)x. Removal of the last amino acid prevents 14-3-3 binding and abolishes surface expression of the channel, indicating that interaction with 14-3-3 is necessary for passage through the secretory pathway. The binding domain for p11 is in the proximal C-terminus of TASK-1 (4); removal of this domain strongly increased surface expression of TASK-1, indicating that binding of p11 causes retention/retrieval in the endoplasmic reticulum (ER). Live-cell imaging of GFP-tagged TASK-1 channels in transfected COS-7 cells showed that after 24 h wild-type TASK-1 was mainly localised to the ER, whereas TASK-1 mutants that were unable to interact with p11 were localised to the surface membrane. We found that ER-localisation of TASK-1 is mediated by a di-lysine retention signal,(K/H)xKxxx, at the C-terminus of p11 (4). Thus, p11 may act as a ‘retention factor’: binding of p11 to the channel causes ER-retention/retrieval. Our results suggest that surface expression of TASK-1 requires either dissociation of p11 or masking of the retention signal on p11. Efficient ER export of correctly assembled ion channels is often achieved by masking of retention signals in one of the membrane protein subunits during the assembly process. The recruitment of a cytosolic retention factor is a second mechanism for posttranslational regulation of surface expression.
University College London 2006 (2006) Proc Physiol Soc 3, SA8
Research Symposium: Posttranslational regulation of surface expression of the K2P channels TASK-1 and TASK-3: role of ER-export signals, retention signals and accessory proteins
Jurgen Daut1
1. Physiology, Marburg University, Marburg, Germany.
View other abstracts by:
Figure 1. Topology of the interaction between TASK-1 and p11.
Where applicable, experiments conform with Society ethical requirements.