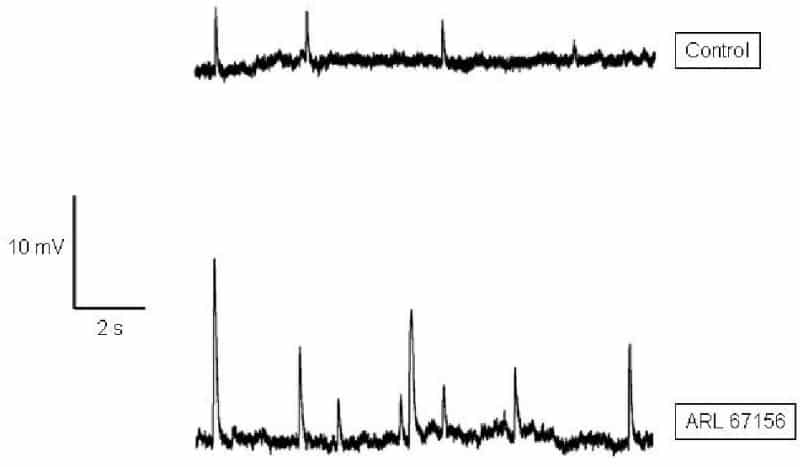
At sympathetic neuromuscular junctions in smooth muscle, ecto-ATPase is believed to curtail the actions of ATP in the synaptic cleft, although details of its modulation of purinergic transmission are not yet clear. An accepted method of evaluating the role of transmitter inactivation is to assess its effect on synaptic potentials following inhibition of the transmitter removal mechanism. Here, we have studied the role of ecto-ATPase in purinergic transmission using ARL 67156, a selective inhibitor of the enzyme. Intracellular recordings of spontaneous excitatory junction potentials (SEJPs), which reflect the underlying transmitter-activated conductance changes (Tomita, 1975), were made from isolated vasa deferentia isolated from humanely killed guinea pigs. 100 μM ARL 67156 significantly increased the mean amplitude, rise time and decay time constant of SEJPs (Student’s t test; P < 0.01), as expected from analogy with inactivation of transmitter-degrading enzymes at other synapses. Interestingly, however, the frequency of occurrence of SEJPs was found markedly to increase (Fig. 1), from 0.28 ± 0.13 Hz in control to 0.90 ± 0.26 Hz in the presence of ARL 67156 (n = 4 cells each; Student's t test; P < 0.01). Although the inter-event interval (IEI) for SEJPs decreased significantly in the presence of ARL 67156 (K-S test; P < 0.01), its distribution remained exponential, indicating that the quantal release events continued to be random. An increase in frequency of spontaneous potentials indicates a pre-synaptic effect of ecto-ATPase inhibition (Khakh, 2000). To probe this possibility further, discrete events (DEs), which represent stimulation-evoked quantal release underlying the excitatory junction potential (EJP), were analysed. As seen for SEJPs, DE frequency increased from 0.7 per EJP in control conditions to 1.4 per EJP with ARL 67156 (single cell data). Although ARL 67156 did not significantly change DE amplitude, it elicited the appearance of DEs in previously 'silent' latency bands. These observations suggest that the ARL 67156-induced elevation of SEJP frequency could be due to a combination of (i) increased transmitter release from previously active varicosities and (ii) recruitment of previously 'silent' varicosities. The precise mechanism underlying these effects is unclear, but one possibility is the enhanced activation of pre-synaptic P2X receptors by elevated concentrations of synaptic ATP due to ecto-ATPase inhibition, for which we have obtained supporting evidence (P. Ghildyal & D. Palani, unpublished data). In conclusion, ecto-ATPase may modulate not only post-synaptic transmitter action but also its pre-synaptic release, thus suggesting novel control mechanisms in the regulation of purinergic transmission.
University of Oxford (2005) J Physiol 568P, PC41
Poster Communications: Pre-synaptic consequences of ecto-ATPase inhibition: modulation of transmitter release in guinea-pig vas deferens
Manchanda, Rohit; Palani, D; Ghildyal, Para;
1. Biomedical Engineering Group, School of Biosciences & Bioengineering, Indian Institute of Technology-Bombay, Mumbai, Maharashtra, India.
View other abstracts by:
Fig. 1 Typical sEJPs recorded from a single cell. Note both potentiation & increased frequency of sEJPs following ecto-ATPase inhibition.
Where applicable, experiments conform with Society ethical requirements.