Adverse intra-uterine conditions induced by placental dysfunction (Robinson et al. 1983; Block et al. 1984; Gagnon et al. 1997), maternal undernutrition (Hanson & Hoet, 1999) and pregnancy at high altitude (Giussani, 1994) are known to alter basal fetal cardiovascular and endocrine functions. However, little is known about the effects of adverse intra-uterine conditions on the fetal cardiovascular and endocrine responses to further stressful stimuli such as acute hypoxaemia. Hence the present study investigated fetal responses to acute hypoxaemia superimposed upon prevailing and independently occurring hypoxaemia, or acidaemia or hypoglycaemia in fetal sheep during late gestation. All animal procedures were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986.
Under 1-2 % halothane anaesthesia (50:50 O2/N2O) 33 sheep fetuses were instrumented with vascular catheters and with a femoral Transonic flow probe at least 7 days before study. Of these, 19 fetuses were designated as either spontaneously hypoxaemic (SpHPX, n = 8), or acidaemic (SpAC, n = 5) or hypoglycaemic (SpHG, n = 6). The criteria for inclusion was determined as one parameter only being -2 S.D. below the normal range in arterial pH, PO2, acid/base excess (ABE) or blood glucose in our laboratory. The remaining fourteen fetuses were used as controls. Between 129 and 137 days, all fetuses were subjected to 1 h of hypoxaemia via maternal inhalational hypoxaemia (9 % O2 in N2). Cardiovascular variables were recorded continuously and fetal blood samples were taken for catecholamine (HPLC) analysis. At the end of the protocol, the ewes and fetuses were humanely put down using a lethal dose of sodium pentobarbitone (200 mg kg-1 Pentoject; Animal Ltd, York, UK)
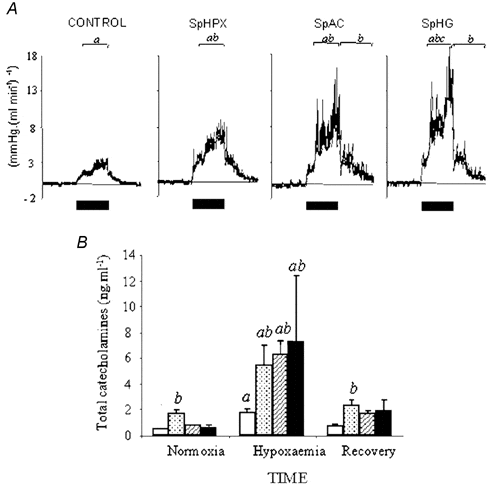
In control fetuses basal arterial pH, PO2, ABE and blood glucose were 7.35 ± 0.01, 23.7 ± 0.8 mmHg, 1.8 ± 0.6 mequiv l-1 and 0.82 ± 0.04 mmol l-1, respectively. In SpHPX fetuses basal PO2 was 16.8 ± 0.9 mmHg; in SpAC fetuses basal pH and ABE were 7.25 ± 0.01 and -4.7 ± 1.4 mequiv l-1, respectively, and in SpHG fetuses basal arterial blood glucose was 0.51 ± 0.04 mmol l-1, P < 0.05 (two-way RM ANOVA) all cases vs. controls. There was no evidence of infection in any experimental group. Acute hypoxaemia reduced fetal PO2 to 12 ± 1 mmHg in all groups. All fetuses responded to acute hypoxaemia with hypertension, femoral vasoconstriction and elevations in catecholamine and vasopressin concentrations. Relative to controls, the increment in blood pressure was greater only in SpHPX fetuses (27 ± 5 vs. 17 ± 2 mmHg), the increment in femoral vascular resistance and increase in plasma catecholamines during hypoxaemia were greater in all compromised groups (Fig. 1A and B).
These data show that fetal cardiovascular and endocrine defences to acute hypoxaemia are modified by adverse intra-uterine conditions but the partial contributions of prevailing fetal hypoxaemia or acidaemia or hypoglycaemia to these modifications can vary. These data have important implications to obstetric practice during labour in pregnancies complicated with abnormal fetal oxygen, acid/base or metabolic status.
This work was funded by the British Heart Foundation.
 View larger image [new window] |
| Figure 1. Data are means ± S.E.M. of femoral vascular resistance (FVR; mmHg (ml min)-1) for 1 h epochs (bars denote period of hypoxaemia; A), or total plasma catecholamine (B) concentration during 1 h of hypoxaemia in control (open columns), SpHPX (stippled columns), SpAC (hatched columns) or SpHG (filled columns) fetuses. Statistical differences are: a, P < 0.05, normoxia vs. hypoxaemia or recovery; b, P < 0.05, SpAC/SpHG vs. control fetuses; c, P < 0.05, SpHPX vs. SpAC fetuses. |