Intro: Fetal growth restriction (FGR) affects 10% of pregnancies globally. FGR results from chronic fetal hypoxia which promotes oxidative stress in utero and increases the risk of chronic disease in later life (1). These chronic diseases generally require long-term drug treatment where tight control of drug-plasma concentrations within a therapeutic range is essential. Hepatic cytochrome P450 (CYP) enzymes metabolise 70-80% of all clinical drugs – with CYP3A metabolising more than 30% – and their activity is impacted by acute and chronic changes in oxygen and glucose availability (2). Understanding programmed changes in hepatic CYP activity can improve therapeutic outcomes; however, programming of hepatic drug metabolism in hypoxic pregnancy has not been investigated. We have shown that hepatic CYP3A activity is reduced in 21d old lambs born FGR (3), although what is driving this alteration is unknown. Mitochondria-targeted antioxidant treatment (MitoQ) in FGR animal models protects against programmed hypertension in adult offspring (4). Whether this protection extends to the programming of altered CYP activity in hypoxic pregnancy has not been investigated.
Aim: To determine if hypoxic pregnancy impairs hepatic CYP activity in offspring and if maternal MitoQ treatment is protective.
Methods: At 100±1 day of gestational age (dGA; term is 145 days) Welsh mountain ewes carrying singletons were catheterized under general anaesthesia (1.5-2.5mg/kg IV alfaxalone, Alfaxan; maintained with 1.5-2% isofluorane in 60:40 O2:N2O) with analgesic administered prior to surgery (1.4 mg/kg SC carprofen). Ewes were randomly allocated to normoxic (21% O2: n=34) or hypoxic (isobaric chambers, 11% O2: n=36) pregnancy with MitoQ (MS010 IV, 6mg/kg) or control (saline IV) treatment from 105-138 dGA. Ewes carrying male fetuses were humanely killed with an overdose of sodium pentobarbitone (0.4ml/kg IV, Pentoject) at ~138 dGA. Ewes carrying female fetuses lambed spontaneously and offspring were humanely killed (as per ewes) at 9 months of age (sexual maturity). Activity of 3 and 7 CYP enzymes in fetal and adolescent offspring respectively was determined in isolated hepatic microsomes using established functional assays (5). Data are presented as mean ± SEM and analysed using two-way ANOVA with the Tukey post hoc test.
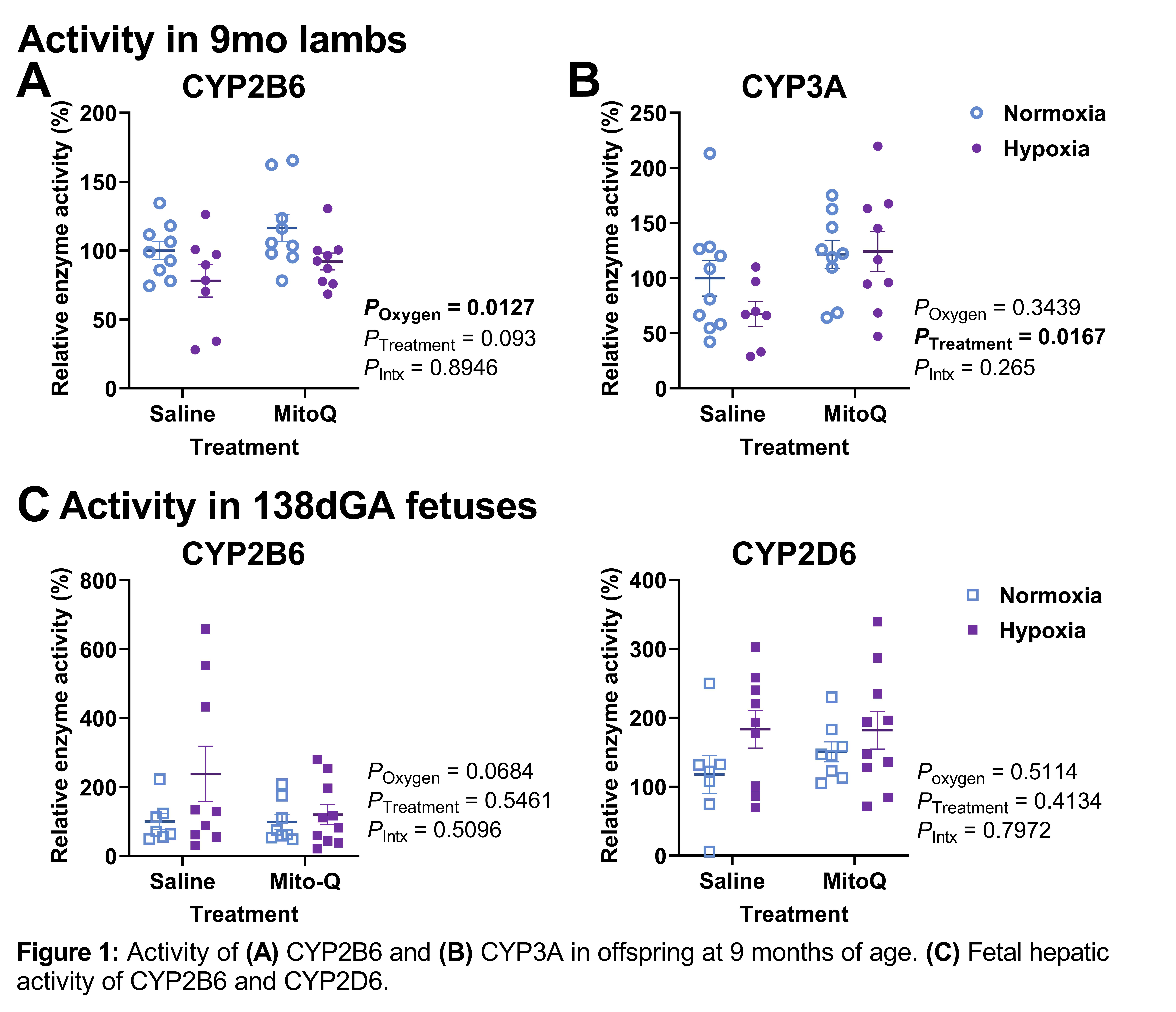
Results: In 9-month-old lambs, hepatic CYP2B6 and CYP2E1 activity was reduced by 22% (Poxygen=0.0127) and 38% (Poxygen=0.0349) respectively in hypoxic pregnancy with no treatment. MitoQ treatment alone in pregnancy significantly decreased CYP1A2 activity by 12% (Ptreatment=0.0011) and CYP3A activity increased by 22% (Ptreatment=0.0167). Conversely, in late-gestation fetuses, no significant alterations in CYP activity were observed in any group.
Conclusions: The fetal data are consistent with many CYPs not becoming active until after birth. Hypoxaemia in late pregnancy programmed reduced CYP activity in adolescent offspring, although unchanged CYP3A activity was unexpected and contrasts previous work potentially due differences in the timing and duration of the hypoxic insult. Despite MitoQ treatment offering protective benefits for hypertension in FGR offspring, our data suggests MitoQ programs increased CYP3A activity and decreased CYP1A2 activity. Programmed changes to hepatic CYP activity in adolescent offspring may alter the efficacy and safety of commonly used therapeutics throughout the life-course.