Mitochondria’s regulation of apoptosis and cellular energy metabolism is influenced by the voltage-dependent anion channel (VDAC) and cytochrome c (Cyt C). The abundance of VDAC is up regulated by maternal nutrient restriction (Mostyn et al. 2003) whilst thymus mass is decreased by maternal under nutrition (Osgerby et al. 2002). This study aimed to determine whether protein supplementation of the maternal diet at defined stages of gestation promoted the growth, development and mitochondrial protein abundance of fetal thymus tissue.
Twenty-nine twin bearing ewes of similar body weight and parity were randomly allocated into four groups from 10 d gestation. Controls were fed a standard diet of chopped hay and barley based concentrate that was increased with gestation. Supplemented groups were randomly allocated to be provided with additional protein in the form of fishmeal (66 % crude protein plus an equal amount of molasses to aid palatability) between either 10-40 d gestation, 40-70 d gestation or from 110 days gestation. All the ewes were humanely euthanised with an overdose of barbiturate (100 mg kg-1 pentobarbital sodium: Euthanal) at 140 d gestation to enable sampling of fetal thymus. Mitochondrial fractions were prepared and VDAC and Cyt C abundance determined by immunoblotting using a polyclonal antibody raised to ovine VDAC and Cyt C. Results are given as means with their standard errors. Statistically significant differences with respect to nutritional supplementation and protein abundance were determined using a General Linear Model (GLM) and Tukey test.
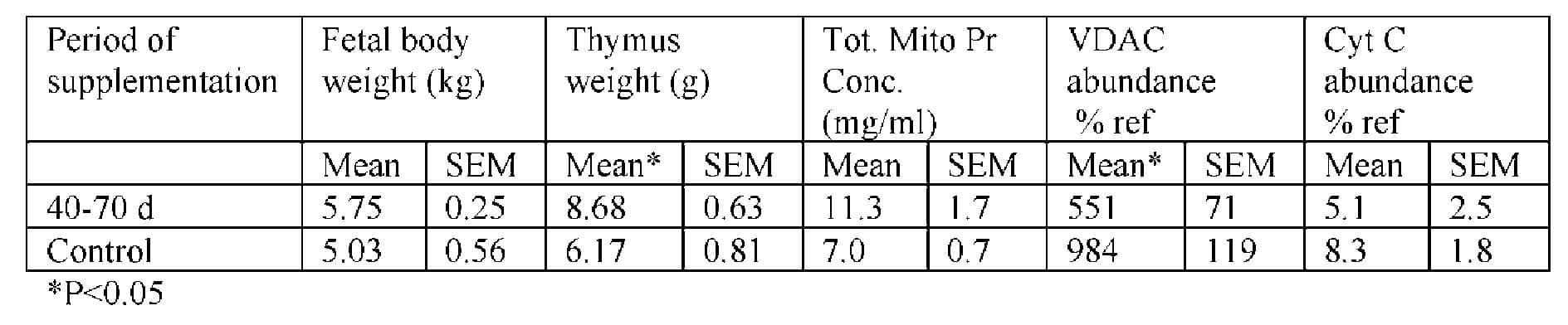
Protein supplementation during mid gestation resulted in larger fetuses with heavier thymuses. The specific mitochondrial VDAC and Cyt C abundance of these thymuses were reduced despite total mitochondrial protein concentration increasing. In conclusion protein supplementation of the maternal diet during the period of maximal placental growth increases thymus growth. This is associated with a decrease in mitochondrial proteins known to regulate energy metabolism. The effects of this on organ function, postpartum remains to be determined.
GH is supported by a BBSRC Postgraduate Studentship. This work was funded by the Bastow Award from the Special Trustees of Nottingham University Hospitals.