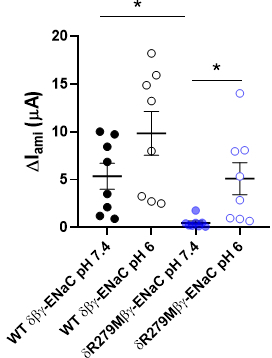
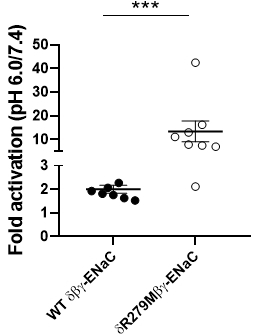
Epithelial sodium channels (ENaCs) are sodium-selective ion channels which regulate salt and water balance in vertebrates. There are four different ENaC subunits (α, β, γ and δ) which can assemble in either the αβγ- or δβγ-combination. The physiological function of δβγ-ENaCs is poorly understood. Using Xenopus laevis as a model, we have recently shown that δβγ-ENaC is profoundly activated by protons, whereas αβγ-ENaC is not (Wichmann et al. 2019). The pH sensitivity of the closely related proton-sensitive ion channel ASIC1a (acid-sensing ion channel 1a) is linked to the amino acid residue K211 which is located in the palm domain between neighbouring channel subunits. We therefore hypothesise that conservation of the positive charge at the corresponding position in Xenopus δ-ENaC (δR279) could account for proton-sensitivity of this ENaC isoform. The absence of the positive charge in the α-ENaC subunit (αM282) may prevent proton-induced channel activation. Site-directed mutagenesis was used to replace the δR279-residue with the corresponding methionine (M) present in the α-ENaC subunit. Both wild-type (WT δβγ-ENaC) and mutant (δR279Mβγ-ENaC) ion channels were expressed in Xenopus laevis oocytes. All procedures involving Xenopus oocytes were approved by the Animal Welfare Ethical Review Board of Newcastle University (ID 630). The activity of the expressed ion channels was measured using the two-electrode voltage-clamp technique at a holding potential of -60 mV. Oocytes were perfused in a Na+ containing solution (Wichmann et al. 2019) at pH 7.4 or pH 6.0. The ENaC inhibitor amiloride (100 µM) was used in order to determine ENaC-mediated current fractions (ΔIami). All data are presented as means ± standard error of the mean. At pH 7.4 (Figure 1), ΔIami in oocytes expressing WT δβγ-ENaC were 5.35 ± 1.36 µA (n = 8) and were significantly larger than in those expressing δR279Mβγ-ENaC (0.48 ± 0.19 µA, n = 8, p < 0.05, one way ANOVA followed by Dunn’s multiple comparison test). The ΔIami of both channels were increased under pH 6.0 (Figure 2). Compared to pH 7.4, pH 6.0 activated δR279Mβγ-ENaC by 13.42 ± 4.42 fold (n = 8), whereas WT δβγ-ENaC was only activated by 2.01 ± 0.17 fold ( n = 8, p < 0.001, Mann-Whitney U-test). In conclusion, δR279Mβγ-ENaC showed a reduced activity at neutral pH but was significantly stronger activated by protons compared to wild type δβγ-ENaC. This suggests that the δR279-residue is important for proton-sensitivity of δβγ-ENaC.
Future Physiology 2019 (Liverpool, UK) (2019) Proc Physiol Soc 45, PC79
Poster Communications: Proton-mediated activation of epithelial sodium channels is affected by the presence of a positively charged amino acid residue in the palm-domain of the δ-subunit
T. Curless1, M. Althaus1
1. School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom.
View other abstracts by:
Figure 1: Amiloride-sensitive currents (?Iami) of oocytes expressing wild-type (WT) dß?-ENaC and dR279Mß?-ENaC mutant at pH 6.0 and 7.4. * p = 0.05.
Figure 2: Fold-activation of WT dß?-ENaC and dR279Mß?-ENaC as calculated from ?Iami at pH 6.0 and pH 7.4.
Where applicable, experiments conform with Society ethical requirements.