Cardiovascular disease is a leading cause of morbidity and hospitalisation at a large financial cost to society and an emotional burden to individuals (Amini et al., 2021). Electrical dysfunction, where the normal rhythm of the heart is disrupted, is a major cause of this morbidity. Therefore it is essential that channel kinetics and the interplay between channels is fully understood as to the role they play in regular and diseased states, including atrial fibrillation.
Computational models of the heart are continuing to grow in sophistication and accuracy and have been a valuable tool for understanding the functional implications of ion channel kinetics. There are multiple contemporary computational models that describe human atrial cellular electrophysiology, that all exhibit different action potential (AP) morphologies underlain by fundamental differences in the formulation and relative expression many ion channels. These differences in part reflect inter-cellular and inter-subject heterogeneity but are also related to inter-laboratory variability and how uncertainties have been included in the model. The formulation of two of the major repolarising currents, the rapid and slow delated rectifier currents (IKr and IKs, respectively), is a major uncertainty in these models, due to the significant challenges in measuring the activity of these currents in isolated cellular preparations. Due to the importance of these channel currents for terminal repolarisation, uncertainties in their formulations could have major implications for pro-arrhythmogenic cellular dynamics, such as alternans and early after depolarisations (EADs), and therefore may critically impact the conclusions of modelling studies.
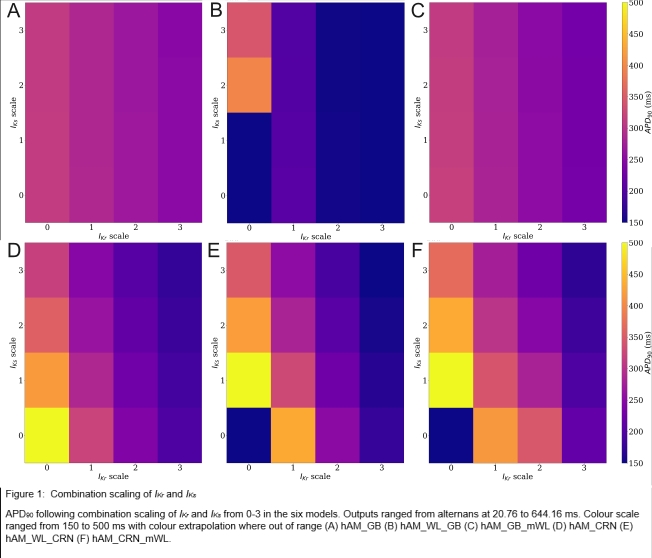
This project aims to quantify the effects of modulating both IKr and IKs in six published mathematical models (Courtemanche et al., 1998; Grandi et al., 2011; Chang et al., 2014; Colman et al., 2018). First, the range of values for the conductance of each current that resulted in AP durations (APD) that fit within physiological ranges was explored. Within these ranges, the impact of variability on APD restitution and alternans was investigated, as well as the interaction with variability in the L-type calcium channel (ICaL).
When scaling both IKr and IKs there was no clear difference was found in APD30. However, there was a large difference, including the presence of alternans, at APD90 in all six models (Figure 1). It was determined that IKr plays a larger role of the two channels with increased prolongation and reduction. For example, a decrease of 37.59 ms at APD90 was found in the original Grandi model compared with IKr scaled to by a factor of three, but only a decrease of 1.11 ms was found with the respective scaling of IKs. Inter-model differences were substantial across the range of behaviour studied, and some behaviours (such as EADs or non-repolarising APs) emerged only in a subset of the models.
In the future there is hope that computational models can be used in clinic to personalise medicines towards a patient’s cellular profile. However, this work highlights the importance of accurately capturing IKr and IKs in fundamental general models of human atrial electrophysiology before truly patient-specific models can be considered.