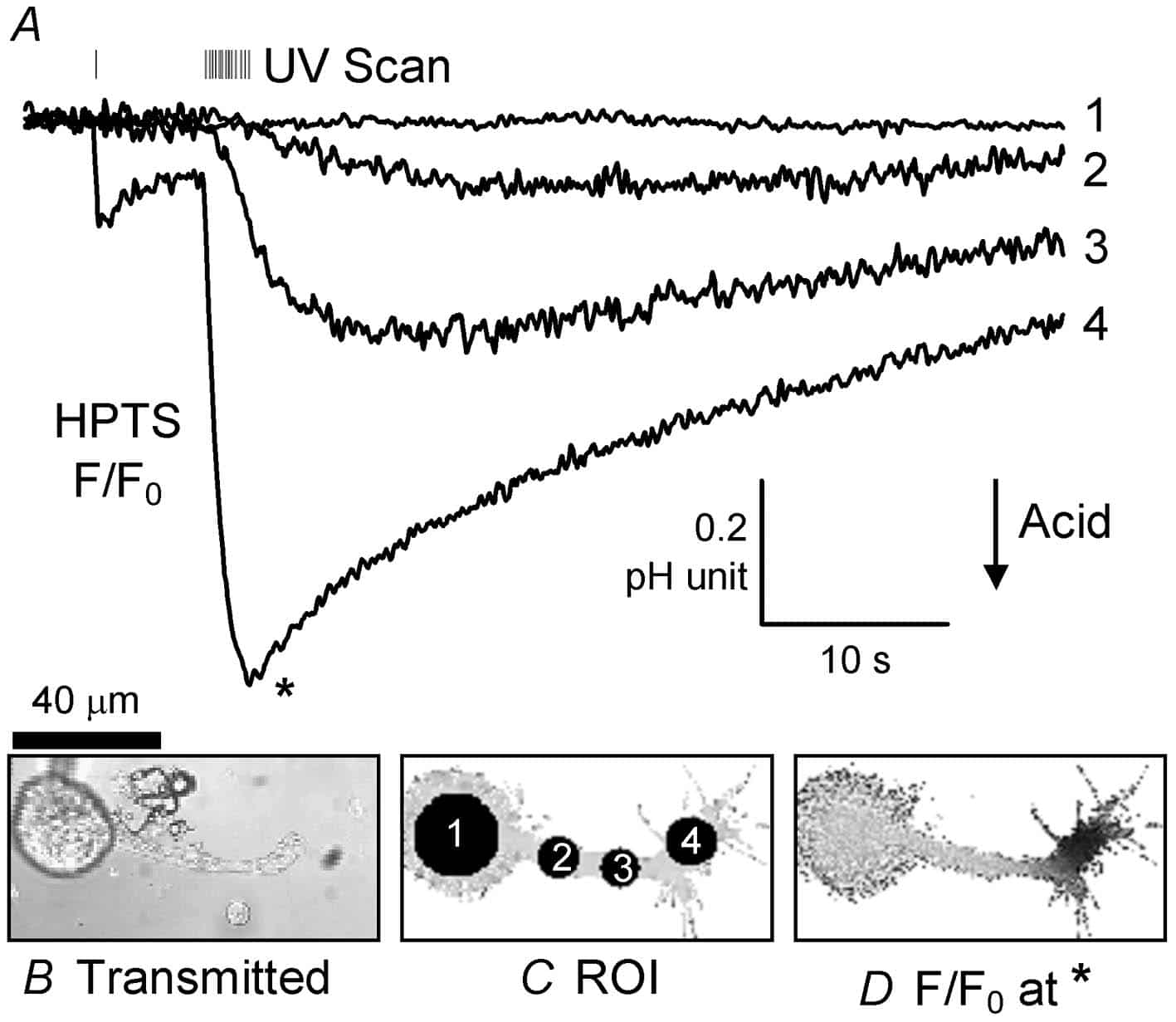
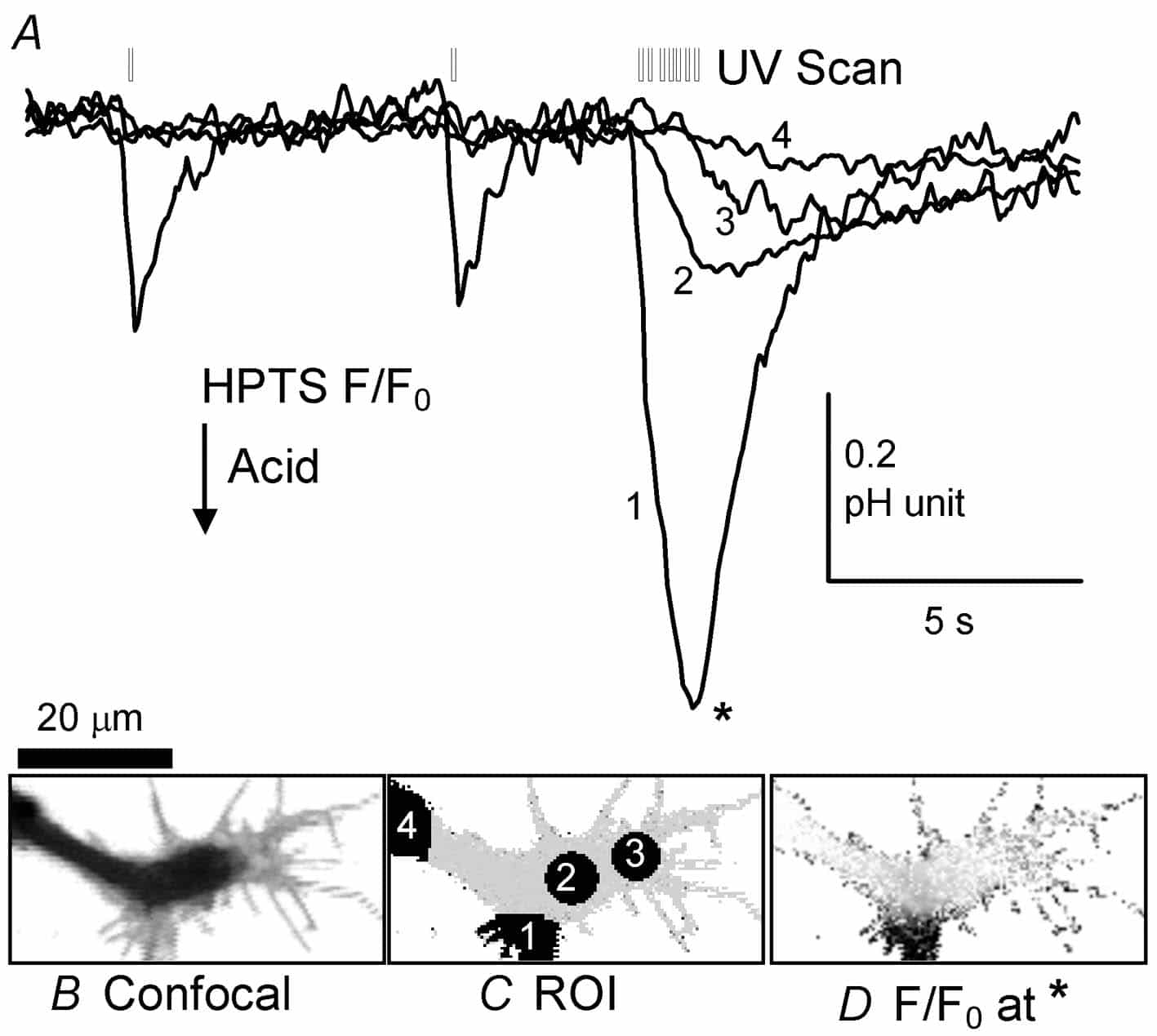
Hitherto three types of techniques have been used to changeintracellular pH (pHi): superfusion of weak acids or bases (Jacobs, 1920), cytosolic injection of acid or alkali, or the activation of channels or transporters (e.g. the Ca2+:H+ pump, Schwiening et al. 1993) that allow transmembrane H+ or HCO3– fluxes. Here I describe a fourth technique, the regional UV-induced release ofprotons (Ciamician & Silber, 1901; George & Scaiano, 1980) that allows for extremely rapid and localized acidic pHi shifts. This technique should provide a simple, cheap and highly controllable way of generating local pHi signals independently of changes in other ion concentrations (e.g. Ca2+).
Helix aspersa neurones were isolated from the gangliafollowing enzymatic digestion and mechanical agitation (Schwiening & Willoughby, 2002). Snail Ringer solution containing the isolated neurones was placed in a superfusion chamber on a Zeiss LSM 510 confocal microscope and the cells were allowed to settle for ~1 h. A cell with an axonal projection was selected and patch clamped in the whole-cell configuration (pipettes ~1.5 µm tip filled with 110 mM CsCl, 0.5 mM of the pH-sensitive dye HPTS). pH-sensitive fluorescence was imaged (488 nm excitation, 505 nm emission) using a 63x water-immersion c-apochromat objective (1.2 NA). In some experiments ratiometric pH recordings were made using alternating 488 nmand 357 nm excitation of the dye HPTS. 2-nitrobenzaldehyde (NBA; Sigma-Aldrich) was bath applied at between 1.0 and 10 mM (made up fresh each day from a 100 mM stock solution in methanol) and uncaged using the LSM510 bleach function (AOTF-modulated illumination with 364 nm light during a whole image sweep) at maximum power.
This demonstration is, to the best of my knowledge, thefirst time acid has been both locally flash released and recorded inside a cell. Such release of intracellular acid is by far the most rapid and controllable method for altering regional pHi. Photolysis of NBA, orits derivatives (Abbruzzetti et al. 2003), should facilitate the investigation pHi microdomain signalling targets in normal neuronalfunction.
I thank the MRC for providing the LSM510 and their high rating of my recent grant applications. However, I deplore their failure to support any of my current work, including that presented here, which has been supported by the University.