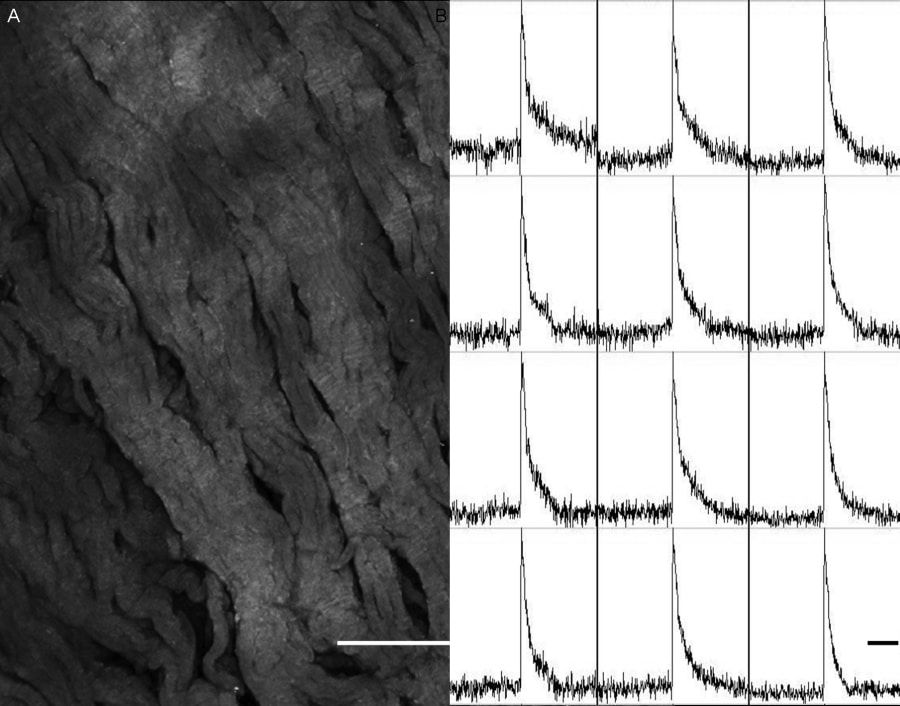
Introduction: Thin tissue slices, cut using a high precision vibratome, are used widely in neuroscience, but so far only a limited number of studies have used them in cardiac research; e.g. Lohmann et al. 2006. Here we present a method for preparing cardiac slices and assess their viability. Methods: Hearts were removed from adult male Wistar rats following schedule 1 killing (according to UK Home Office regulations) and swiftly perfused in Langendorff mode, first with physiological Tyrode, and then with Tyrode containing a fluorescent dye. For optical mapping Tyrode containing 4 µM N-(4-sulphobutyl)-4-(6-(4-(dibutylamino)phenyl)hexatrienyl)pyridinium, (RH237, Invitrogen) was used; for morphological studies Tyrode containing 4 µM pyridinium 4-2-6-(dibutylamino)-2-naphthalenyl)ethenyl)-1-(3- sulphopropyl)-, hydroxide (di-4-ANEPPS, Invitrogen) was used. Following this, part of the left ventricular free wall was removed. Trabeculae were trimmed to create a flat endocardial surface. The tissue segment was attached by the endocardium onto the cutting stage of the vibratome (VT1000 S, Leica), using cyanoacrylate glue (Histoacrylate, Braun). The tissue was submerged into ice-cold Tyrode solution containing 10 mM 2,3-butanedione monoxime (BDM) to prevent contraction. Tissue sections were cut in the plane of the epicardial surface, with a thickness of 350 μm, and stored in BDM-Tyrode at room temperature until use. For optical mapping records were obtained from 800 µm square regions, using a 20x water immersion lens with a 256 element photodiode array, multiple regions of the same slices where imaged to assess viability. Results: This procedure has allowed us to produce tissue slices with cells orientated largely parallel to the sectioning plane (Fig. 1A). Optical mapping confirmed electrical excitability of cells, with clear action potential waveforms (Fig. 1B) present in 89% of slices (8 out of 9, from 2 hearts), and in 58% of tissue regions (22 out of 38) studied. Discussion: While cell cultures are increasingly able to reproduce essential aspects of cardiac structure and function (Camelliti et al. 2006), it remains desirable to obtain more realistic models of cardiac behaviour that can be used across species and functional states. Ventricular slices produced with the above technique may hold promise for improved structure-function investigations.
University of Cambridge (2008) Proc Physiol Soc 11, PC65
Poster Communications: Rat left ventricular wall thin slices as a 3-dimensional cardiac tissue model
G. K. Picton1, P. Camelliti1, G. Bub1, A. Bussek2, E. Wettwer2, U. Ravens2, P. Kohl1
1. Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom. 2. Department of Pharmacology and Toxicology, Medical Faculty, Dresden University of Technology, Dresden, Germany.
View other abstracts by:
Fig. 1. Rat left ventricular tissue slice characteristics. A. Morphology shown by labelling with di-4-ANEPPS (scale bar 200 µm) B. Electrical excitability confirmed by optical mapping (scale bar 200 ms).
Where applicable, experiments conform with Society ethical requirements.