Skeletal muscle fibre type and structural alterations, as seen during regeneration and in certain muscle diseases, can be challenging to interpret. Earlier we have described the spatial and temporal cellular processes occurring in the first 7 days after electrically induced myofibre necrosis in human skeletal muscle (1). Furthermore, we previously reported remnants of regeneration in the myofibre and its basement membrane 30 days post stimulation (2), where changes in the occurrence of small myofibres and encroachment of sarcolemma and basement membrane (suggestive of myofibre branching/splitting) were observed through a serial sectioning approach of the biopsy samples. The purpose of this study was 1) to investigate these phemonena further in a systematic manner, and 2) to determine the reliability of fibre-typing human muscle tissue undergoing regeneration.
Methods: Muscle biopsies from 3 individuals demonstrating muscle regeneration were sectioned serially and stained for ATPase, and antibodies against type I and II myosin, embryonic and neonatal myosin, as well as dystrophin and laminin to label the sarcolemma and basement membrane, respectively. Single fibres and tissue blocks were examined by confocal and electron microscopy, respectively.
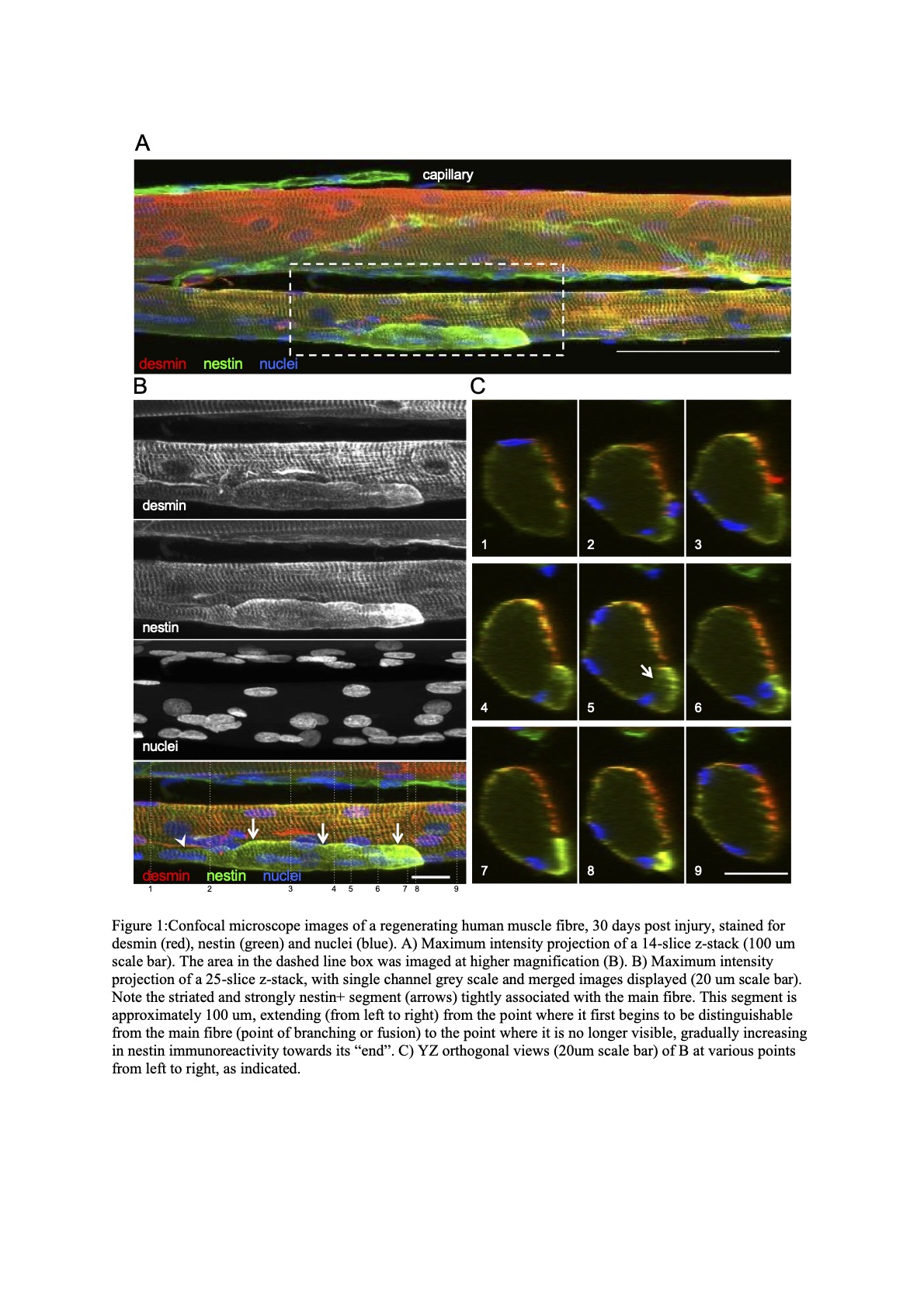
Results: A classification guide of approximately 210 regenerating muscle fibers and 180 control fibres was created, consisting of 8 “fibre type” profiles. Comparing the staining patterns of the control and the regenerating muscle revealed solely type II muscle fibres were affected by electrical stimulation damage. In addition, confocal and electron microscopy images revealed regular branching of small myofibre segments, most of which were observed to fuse further along the fibre. Central nuclei were frequently observed at the point of branching/fusion.
Conclusions: Human muscle fibres expressing embryonic and neonatal myosins complicate the determination of fibre type using traditional methods such as ATPase or type I and II myosin antibodies. So-called myofibre branching or splitting is likely to be explained by incomplete regeneration after a necrosis-inducing event.
Biomedical Basis of Elite Performance 2022 (University of Nottingham, UK) (2022) Proc Physiol Soc 49, PC24
Poster Communications: Regenerating human skeletal muscle: fibre branching and fibre typing
Grith Højfeldt1,2, Abigail L. Mackey1,2, Jesper Andersen1, Michael Kjaer1, Trent Sorenson1,3, Alana Gonzales1,3
1 Institute of Sports Medicine Copenhagen, Department of Orthopaedic Surgery M, Copenhagen University Hospital - Bispebjerg and Frederiksberg 2 Centre for Healthy Aging, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen 3 Middlebury College, Department of Biology
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.