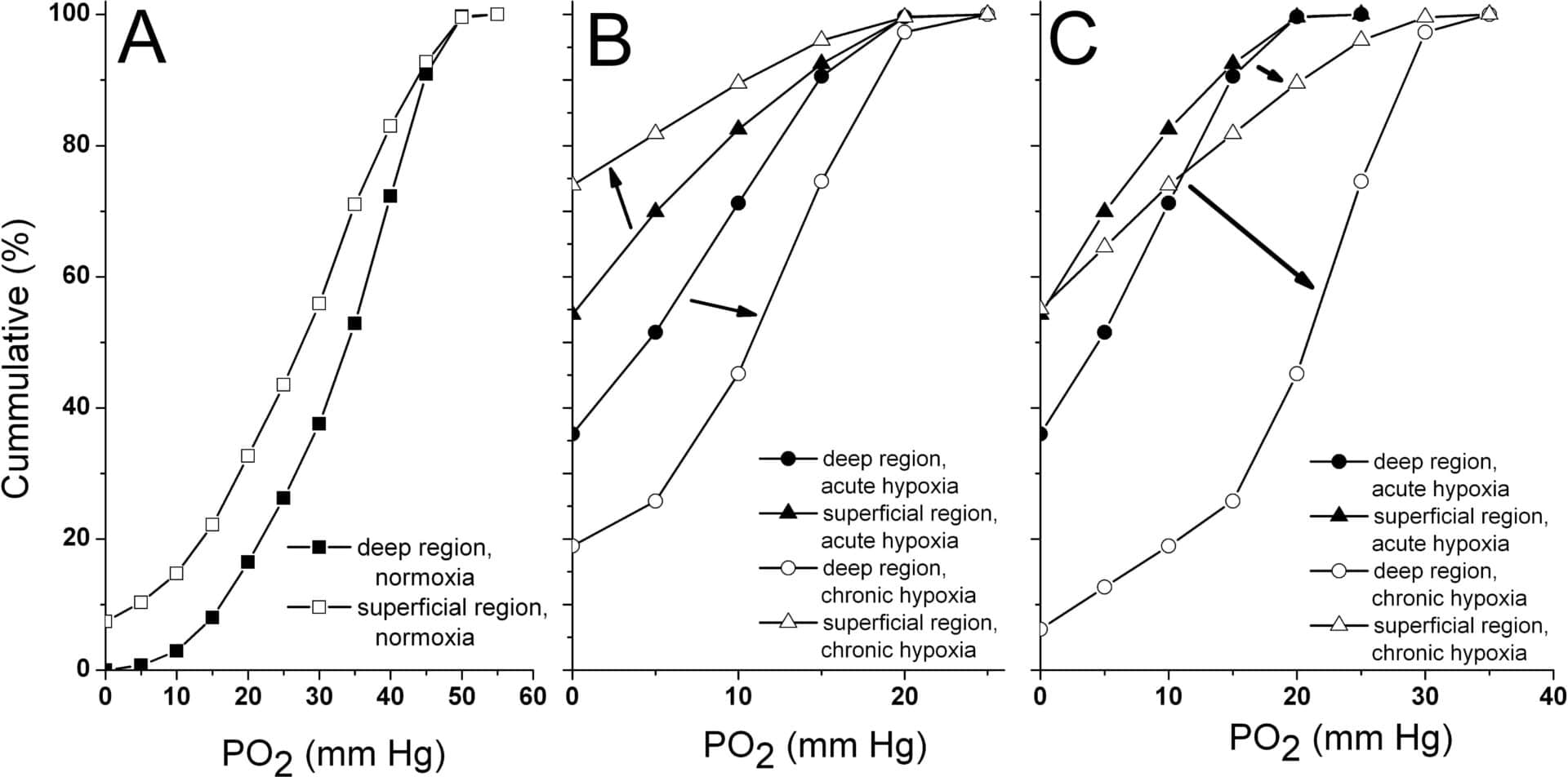
Chronic exposure to hypoxia is often seen in patients with lung and heart failure and is generally associated with muscle atrophy (i.e. reduction in muscle fibre cross-sectional area), reduced oxidative capacity and capillary growth. As some controversy still exists in the literature to what extend these changes are muscle and fibre type specific, we hypothesize that different regions of the same muscle would respond differently to severe chronic hypoxia. To investigate this we compared the deep (oxidative) and superficial (glycolytic) region of the plantaris muscle of 8 male rats exposed to 4 weeks hypobaric hypoxia (410 mm Hg) with those of 9 normoxic rats. The haematocrit was higher in chronic hypoxic than control rats (59 vs. 50%; P<0.001). Using histochemistry, we observed a 10% fiber atrophy (P<0.05, multilevel analysis) in both regions of the muscle, but no shift in fibre type composition and myoglobin concentration of the fibres. In hypoxic rats, the succinate dehydrogenase (SDH)-activity was elevated in fibres of each type in the superficial (25%, P<0.05), but not in the deep region, while in the deep, but not the superficial region, the number of capillaries supplying a fibre was elevated (14%, P<0.05). Model calculations (Krogh model) showed that the region-specific alterations in fibre size, SDH-activity and capillary supply to a fibre prevented the occurrence of anoxic areas in the deep region, but not in the superficial region. Interestingly, the acclimatization-induced increases in mean capillary oxygen pressure (from 25 to 36 mm Hg, [1]) counteracted the impaired tissue oxygenation in the glycolytic region and further improved the tissue oxygenation in the deep region. We conclude that the determinants of tissue oxygenation show region specific adaptations, resulting in a marked differential effect on tissue oxygen tension.
King's College London (2009) Proc Physiol Soc 14, PC46
Poster Communications: Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia
H. Degens1, L. J. Hoofd3, W. J. Van der Laarse4, R. T. Jaspers5, A. F. van Heijst6, R. C. Wüst1,2
1. Institute for Biomedical Research into Human Movement and Health, Manchester Metropolitan University, Manchester, United Kingdom. 2. Institute of Membrane and Sytstems Biology, University of Leeds, Leeds, United Kingdom. 3. Department of Physiology, Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands. 4. Department of Physiology, VU University Medical Centre, Amsterdam, Netherlands. 5. Research Institute MOVE, VU University Amsterdam, Amsterdam, Netherlands. 6. Department of Paediatrics, Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands.
View other abstracts by:
Figure 1: Muscle tissue oxygenation during (A) normoxia (B) acute hypoxia and when taking into account tissue adaptations to chronic hypoxia and (C) chronic hypoxia when also taking into account adaptations in blood flow and haematocrit.
Where applicable, experiments conform with Society ethical requirements.