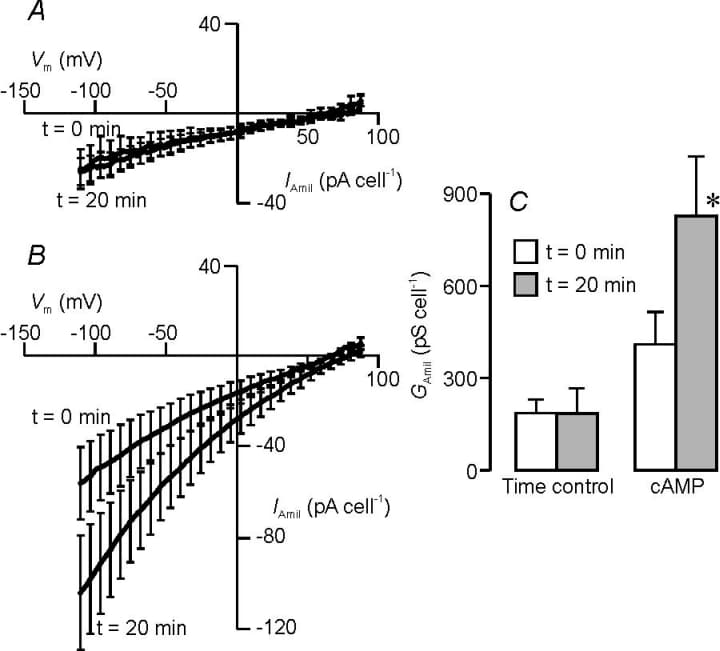
Distal airway epithelia absorb Na+ from the airway surface liquid and this process is acutely regulated by cAMP-dependent agonists (Olver et al. 2004). However, some groups attribute this response to an increase in sodium conductance (GNa,see e.g. Collett et al. 2002) whilst others suggest that such agonists act by hyperpolarizing V m and thus increasing the driving force for Na+ entry (O′Grady et al. 2000). Here we report the effects of cAMP on the Na+ conductance expressed by dexamethasone-stimulated H441 distal airway epithelial cells (Clunes et al. 2004), which has properties essentially identical to the conductance associated with co-expression of α, β and γ-ENaC (Canessa et al. 1994).Exposing the cells to a cocktail of cAMP-activating drugs caused a clear increase in GNa (Fig 1A,C) whilst no such change in Goccurred Na in control cells (Fig 1B,C). The cAMP-activating drugs had no effect upon the reversal potential for the amiloride-sensitive component of the membrane current (control E rev 74 ± 11 cf. cAMP-stimulated E 82 ± 8 mV), and so this increase in conductance occurs with no change in Na+ selectivity. In contrast, the cAMP cocktail caused a depolarising shift in the reversal potential for the total membrane current (P<0.05, ∆E rev 18 ± 4 mV) indicating the response is associated with a depolarisation of Vm.These data thus suggest that cAMP-stimulated Na+ absorption reflects an increase in the activity and or surface abundance of highly selective sodium channels. Figure 1. H441 cells were grown (~24 h) on glass coverslips in the presence of 200 nM dexamethasone before being studied using the perforated patch technique; all data are mean ± S.E.M. (n>4). The current − voltage relationship for the amiloride-sensitive current (IAmil) measured at 0 and 20 min in control (A) or cAMP stimulated cells (B, 10 µM forskolin, 100 µM isobutylmethylxanthine, 1 mM N6,2′-O-dibutyryladenonsine 3′5′-cyclic monophoshate). C, Estimates of the amiloride-sensitive membrane conductance (GAmil) from linear regression of data collected at negative holding potentials at 0 and 20 min. * denotes significance P<0.05 (Student’s t test).
University of Glasgow (2004) J Physiol 557P, C50
Communications: Regulation of a selective Na+ conductance in H441 airway epithelial cells by cAMP
M.T. Clunes, A.G. Butt and S.M. Wilson
Division of Maternal and Child Health Sciences, University of Dundee, Dundee, Scotland, UK and Department of Physiology, University of Otago, Dunedin, New Zealand
View other abstracts by:
Figure 1. H441 cells were grown (~24 h) on glass coverslips in the presence of 200 nM dexamethasone before being studied using the perforated patch technique; all data are mean ± S.E.M. (n>4). The current − voltage relationship for the amiloride-sensitive current (IAmil) measured at 0 and 20 min in control (A) or cAMP stimulated cells (B, 10 µM forskolin, 100 µM isobutylmethylxanthine, 1 mM N6,2′-O-dibutyryladenonsine 3′5′-cyclic monophoshate). C, Estimates of the amiloride-sensitive membrane conductance (GAmil) from linear regression of data collected at negative holding potentials at 0 and 20 min. * denotes significance P<0.05 (Student’s t test).
Where applicable, experiments conform with Society ethical requirements.