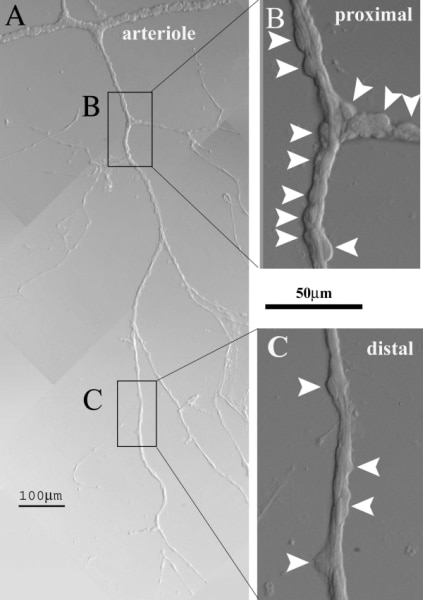
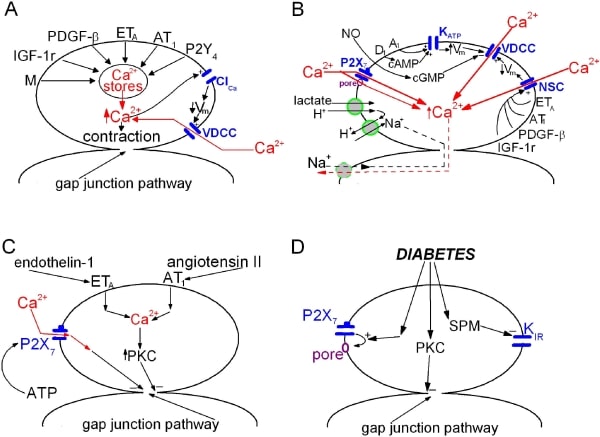
Evidence is accumulating that pericyte-containing microvessels, which constitute the largest component of the circulatory system, actively regulate local perfusion. Because the retinal vasculature is specialized for the local control of blood flow, study of its microvessels is proving useful in the quest to elucidate the mechanisms by which local perfusion is regulated. The microcirculation of the retina is also a focus of attention due to its vulnerability to diabetes, which is a leading cause of vision loss. This talk will give an overview of our recent experimental findings with an emphasis on unexpected observations and new ideas concerning the regulation of retinal microvascular function (1). We use perforated-patch pipettes to monitor ionic currents, fura-2 to measure calcium levels and time-lapse photography to visualize changes in mural cell contractility and lumen diameter in microvessels freshly isolated from the adult rat retina. For the study of diabetes, rats are injected with streptozotocin. To isolate microvascular complexes, retinas were rapidly removed post mortem and then incubated in a papain-containing solution. Subsequently, a retina is gently sandwiched between two glass coverslips; adhering to the coverslip contacting the vitreal side of the retina are complexes that include smooth-muscle encircled arterioles and pericyte-containing capillaries (Fig. 1). Recently, we began to assess how putative vasoactive signals regulate ion channels, intracellular calcium and mural cell contractility (Fig. 2). Unexpectedly, we found that in addition to evoking the release of stored calcium, activating non-specific cation channels and causing pericyte contraction, angiotensin II, ATP and endothelin-1 potently inhibit cell-to-cell communication within pericyte-containing microvessels. This regulation of gap junction pathways is a newly appreciated mechanism by which extracellular molecules regulate microvascular function. Indicative of the importance of gap junction-mediated transmission, our recent study of the electrotonic architecture of the pericyte-containing microvasculature revealed that locally induced voltage changes are efficiently transmitted throughout a vascular network. As a result, a localized exposure to angiotensin, ATP or endothelin initially evokes depolarization throughout a microvascular complex. However, when gap junctions close, intercellular transmission ceases. Thus, it appears that the spatiotemporal dynamics of the microvasculature’s response to extracellular signal may be more complex than previously thought. Another unexpected observation was that pericyte-containing retinal microvessels express P2X7 purinoceptors, whose activation causes vasoconstriction (Fig. 2), but also results in the formation of large transmembrane and the triggering of microvascular cell death. Interestingly, soon after the onset of diabetes, the concentration of P27 needed to open pores and to trigger apoptosis decreases by approximately 80-fold. This increased formation of pores appears to be due to a diabetes-induced enhancement of the transition from activated P2X7 receptor/channels to opened pores. In this way, normally non-lethal concentrations of P2X7 ligands may trigger cell death in microvessels of the diabetic retina. In other studies, we detected a topographical heterogeneity of KIR currents. At distal sites within pericyte-containing retinal microvessels, the KIR current shows strong inward rectification, but proximally the KIR current is only weakly rectifying. Because of the efficient electrotonic transmission within retinal microvessels, the outward current of the proximal KIR channels plays a significant role in setting the membrane potential of cells located throughout the microvasculature. Of potential pathobiological significance, KIR heterogeneity is lost soon after the onset of diabetes, as rectification of the proximal KIR current increases. This diabetes-induced effect is reversed by an inhibitor of polyamine synthesis and is mimicked by spermine, whose concentration is elevated in the diabetic eye. These new findings raise the possibility that functional changes in KIR channels contribute to the dysregulation of blood flow detected early in the course of diabetic retinopathy. Recent studies reveal previously unrecognized mechanisms by which the function of the retinal microvasculature may be regulated in health and disease.
Queen's University Belfast (2007) Proc Physiol Soc 7, SA11
Research Symposium: Retinovascular physiology and pathobiology: New approaches, new ideas
D. G. Puro1
1. Departments of Ophthalmology and Physiology, University of Michigan, Ann Arbor, MI, USA.
View other abstracts by:
Figure 1. A vascular complex freshly isolated from the retina of an adult rat.
Figure 2. Schematic summaries of recently characterized mechanisms mediating the response of retinal microvessels to extracellular signals.
Where applicable, experiments conform with Society ethical requirements.