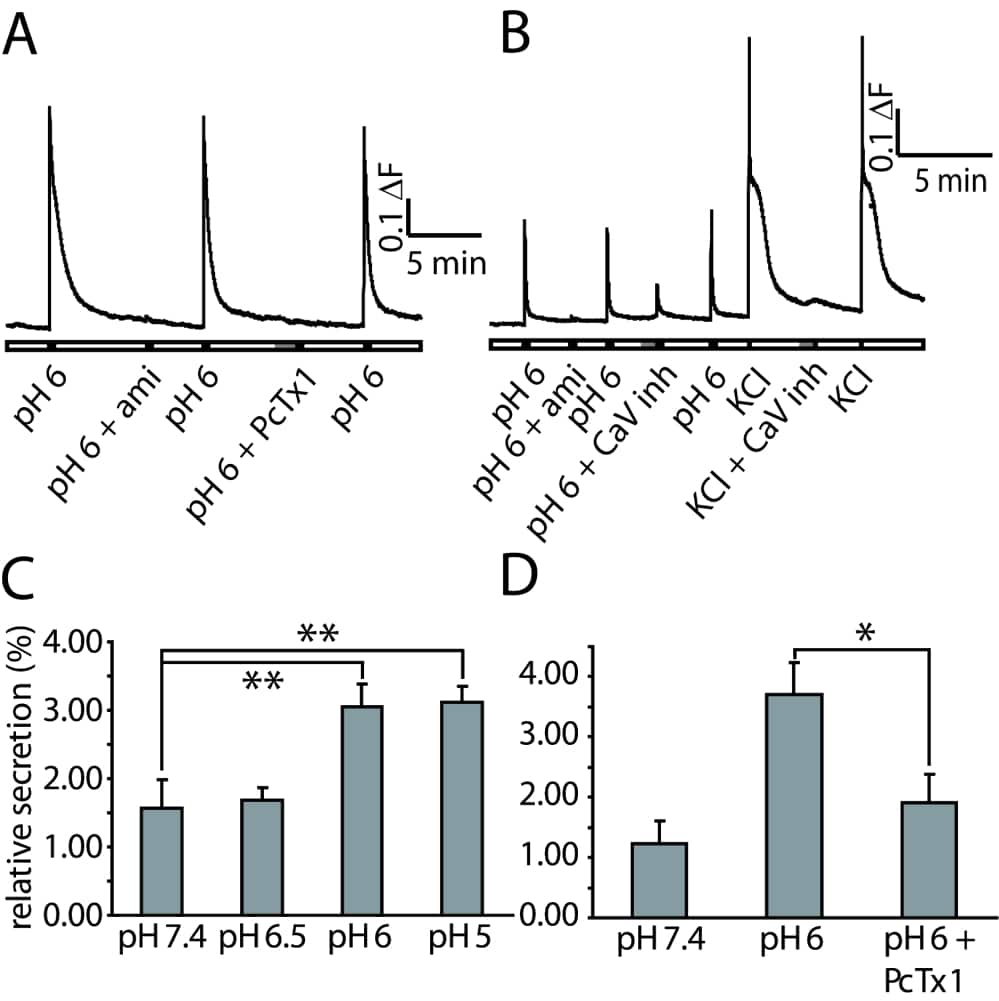
Acid-sensing ion channels (ASICs) are non-voltage-gated sodium channels activated by an extracellular acidification. They are widely expressed in neurons of the central and peripheral nervous system. Tissue damage leads to the release of inflammatory mediators. This so called inflammatory soup includes, among different signalling molecules, serotonin, histamine, glutamate, ATP and protons. Isolectin B4-negative neurons are able to release neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP). Neurogenic inflammation refers to the process whereby peripheral release of the neuropeptides, CGRP and SP, induces vasodilation and extravasation of plasma proteins, respectively. Our laboratory has previously shown that calcium-permeant homomeric ASIC1a channels are present in a majority of IB4 negative, CGRP- and SP-positive small diameter sensory neurons (Poirot et al., 2006). We hypothesize that a local acidification can produce an ASIC-mediated calcium-dependant neuropeptide secretion. To test this hypothesis we have first verified the co-expression of ASIC1 and CGRP/SP using immunochemistry on dissociated rat dorsal root ganglion (DRG) neurons. We found that most CGRP/SP-positive neurons also expressed ASIC1a subunits. Calcium imaging experiments with fura-2 AM dye showed that an extracellular acidification can induce an increase of intracellular Ca2+ concentration, which is essential for secretion. This increase of intracellular Ca2+ concentration is, at least in some cells, ASIC dependant, as it can be prevented by Amiloride, a non-specific ASIC antagonist and by Psalmotoxin (PcTx1), a specific ASIC1a antagonist. Voltage-gated calcium (CaV) channels also play an important role. CaV inhibitors (ω-conotoxin MVIIC, Mibefradil and Nifedipine) reduce the Ca2+ increase in amiloride sensitive neurons during an acidification to pH 6. Secretion assay results show that CGRP secretion can be induced by extracellular acidification in cultured rat DRG neurons. PcTX1 reduced the acidification-induced CGRP secretion, suggesting that homomeric ASIC1a may play a key role in this process. Taken together, these results show that in our in vitro model a mild extracellular acidification can induce a calcium-dependant neuropeptide secretion. In conclusion, in a subpopulation of DRG neurons, this secretion is mediated by ASICs, and especially ASIC1a channels, even if we can not totally exclude the involvement of TRPV1, the other major acid sensitive channel present in DRG neurons.
Durham University (2010) Proc Physiol Soc 21, C01 and PC01
Oral Communications: Role of Acid-sensing ion channels (ASICs) in neuropeptide secretion from sensory neurons.
A. Boillat1, S. Kellenberger1
1. Department of Pharmacology and Toxicology, University of Lausanne, Lausanne, Switzerland.
View other abstracts by:
Extracellular acidification increases intracellular Ca2+ concentration and CGRP secretion. A-B: Calcium imaging experiments in rat DRG neurons. A: Amiloride (500 μM) and PcTx1 (10 nM) prevent intracellular calcium increase by extracellular acidification. B: Voltage-gated calcium channel inhibitors decrease acidification- induced Ca2+-entry. C-D: CGRP secretion assay in cultured rat DRG neurons at 37°C, 15 minutes of secretion. Quantity of CGRP is measured with an enzyme immunoassay. Values are expressed relative to the total cellular CGRP content. C: extracellular acidification increases CGRP secretion. D: PcTx1 (10 nM) inhibits CGRP secretion activated by extracellular acidification. Statistics: **:P<0.01, *:P<0.05, unpaired t-test.
Where applicable, experiments conform with Society ethical requirements.