Increased cardiac output in fish is achieved largely by an increase in stroke volume. This is achieved by an increased end-diastolic ventricular volume and a stretch-induced increase in myocardial contractility known as the Frank-Starling mechanism (Farrell & Jones, 1992). Compared to mammalian myocardium, relatively few details of this mechanism are known for fish.
Trout (159 ± 9 g, mean ± S.E.M.) were killed humanely. Single ventricular myocytes were isolated enzymatically then placed in the chamber of an inverted microscope and superfused at 21-22 °C with a physiological saline solution containing 2 mM Ca2+ (pH = 7.8). Carbon fibre transducers were attached towards either end of the myocyte about 100 µm apart, in order to record tension and to apply axial stretch to the longitudinal axis of the myocyte. Stretch was measured as increased sarcomere length (SL) between the fibres (Hongo et al. 1996). Cells were stimulated by external electrodes at 0.5 Hz and contracted auxotonically.
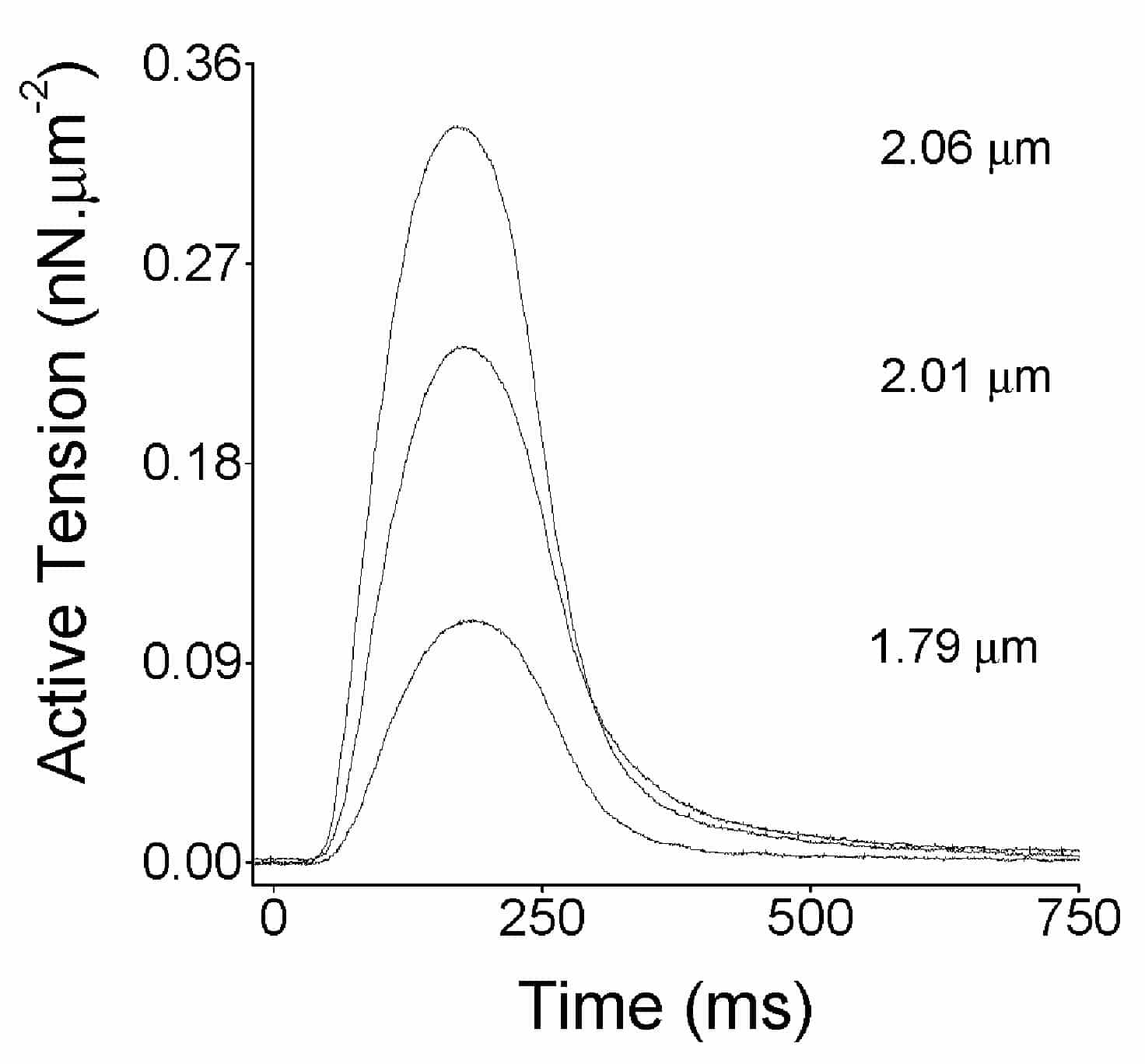
The mean end diastolic SL was (1.84 ± 0.02 Ám, n = 19 myocytes from 6 hearts) at slack length. When cells were progressively stretched there was a progressive increase in both the passive tension (during diastole) and the active tension (additional tension developed during systole, Fig. 1). Analysis of stretches in 19 cells predicted each 1% increase in SL above slack SL caused approximately a 20% increase in active tension (linear regression, R = 0.75, P < 0.001).
These are the first data measuring SL in contracting fish myocytes and demonstrating the Frank-Starling mechanism in single fish cardiac myocytes. Resting SL was similar to that typically reported in mammalian myocytes. The slope of our SL-tension relationship is consistent with data from trout cardiac muscle strips (Harwood et al. 1998) where active stress increased by approximately 350 % when length was increased from 70 to 90 % Lmax (the length at which maximum active stress is recorded).
This work was supported by NSERC Canada and The University of Leeds.