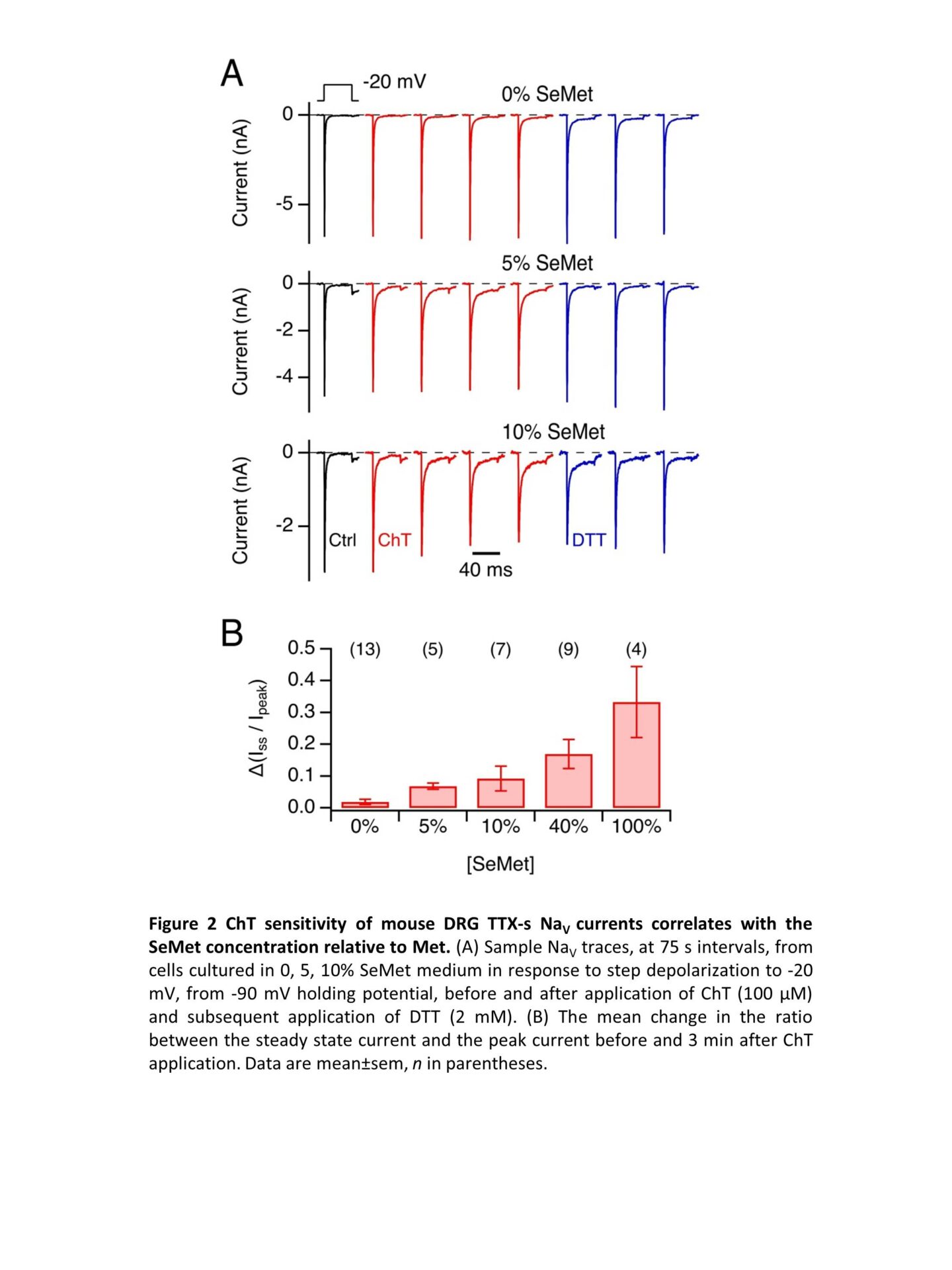
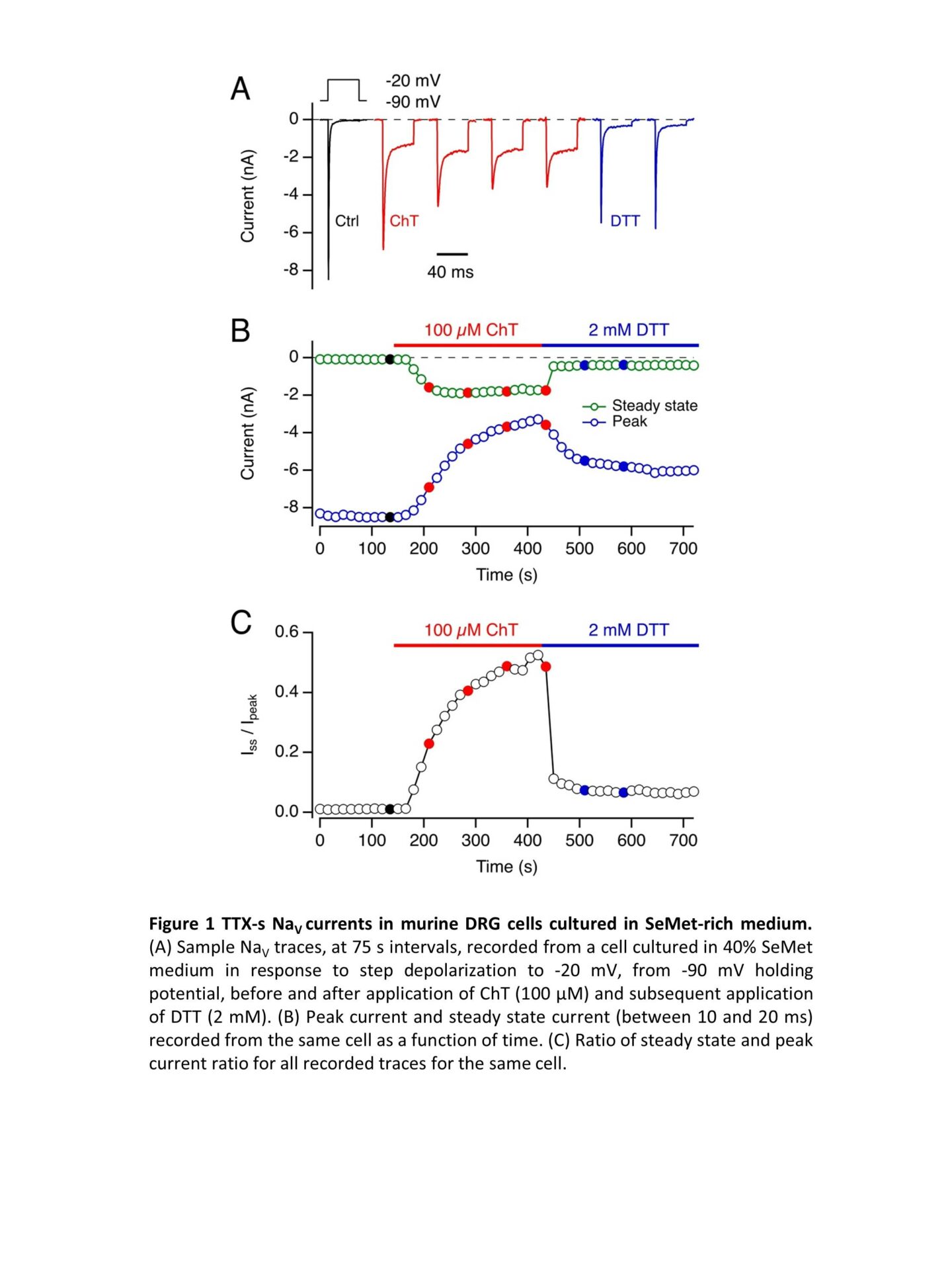
Selenomethionine (SeMet) is widely marketed as anti-aging food supplement. Due to its structural similarity to methionine (Met), SeMet is loaded on Met tRNA and is efficiently incorporated into proteins during translation. Unlike Met, SeMet is readily oxidized; furthermore, reduction of methionine selenoxide occurs non-enzymatically, e.g. in the presence of reducing agents such as dithiothreitol (DTT). Met oxidation in voltage-gated sodium channels (NaV channels) has a gain-of-function effect as it leads to impairment of fast inactivation (1). Even small gain-of-function changes in neuronal NaV channels can cause neuronal hyperexcitability and contributes to the pathology of, e.g., epilepsy and erythermalgia (2). When skeletal muscle NaV1.4 channels are heterologously expressed in a SeMet-rich medium, they incorporate SeMet which makes them sensitive to oxidation. Here we address the question if and to which degree SeMet is incorporated into neuronal NaV channels in native neurons and how this might alter their function. We chose mouse dorsal root ganglia (DRG) nociceptor neurons because of their relevance for pain signaling, their likely exposure to oxidative stress, and their rich repertoire of tetrodotoxin-sensitive (TTX-s) (mostly NaV1.3, NaV1.6, NaV1.7) and TTX-resistant NaV channels (NaV1.8, NaV1.9). Because the inactivation of TTX-r channels is markedly slower than that of TTX-s channels, we employed a double-knockout mouse model (Scn10a/11a–/–), lacking functional NaV1.8 and NaV1.9, to gain access to TTX-s NaV currents in isolation. Wild type (C57BL/6J) and Scn10a/11a–/– mice were sacrificed according to protocols approved by the local animal welfare committee. DRGs were extracted, neurons were separated by enzymatic digestion and seeded on poly-L-lysine-coated glass cover slips in high-glucose Gibco Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 4% penicillin/streptomycin. Met concentration in this medium was about 200 µM. To introduce SeMet to the cultures, Met-free medium with a SeMet concentration of 150 µM was prepared. 18 to 24 h after seeding the cells, the culture medium was partially replaced with the SeMet-containing medium to achieve a SeMet fraction of 0, 5, 10, 40, or 100% of the combined Met and SeMet molarity. Whole-cell patch-clamp measurements were performed 16-24 h later. Even with 100% SeMet, cells from both strains were viable at the time for measurements and showed functional expression of NaV channels. TTX (1 µM) blocked NaV currents of Scn10a/11a–/–. In DRGs from Scn10a/11a–/– mice under 40% SeMet conditions, the Met oxidant chloramine T (ChT, 100 µM) markedly impaired fast inactivation and diminished the maximal current amplitude, both effects reversible under 2 mM DTT (Fig. 1). Even SeMet fractions of only 5% resulted rendered TTX-s channels sensitive to oxidation to yield measurable sustained currents (Fig. 2). The reversibility of ChT-mediated changes by DTT in a clear indication of SeMet incorporation into the channel proteins demonstrating that the protein turnover in DRG neurons occurs on the order of several hours. The observed oxidation-dependent gain of function in even low SeMet concentrations suggests a possible involvement for SeMet mis-incorporation in SeMet toxicity and motivates investigations into how high-SeMet diet might affect neuronal excitability.
Physiology 2021 (2021) Proc Physiol Soc 48, OC24
Oral Communications: Selenomethionine mis-incorporation in TTX-sensitive voltage-gated sodium channels of mouse dorsal root ganglia
Marwa Ahmed1, Rama Hussein1, Stefan H. Heinemann1
1 Center of Molecular Biomedicince, Department of Biophysics, Friedrich Schiller University Jena and Jena University Hospital, Jena, Germany
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.