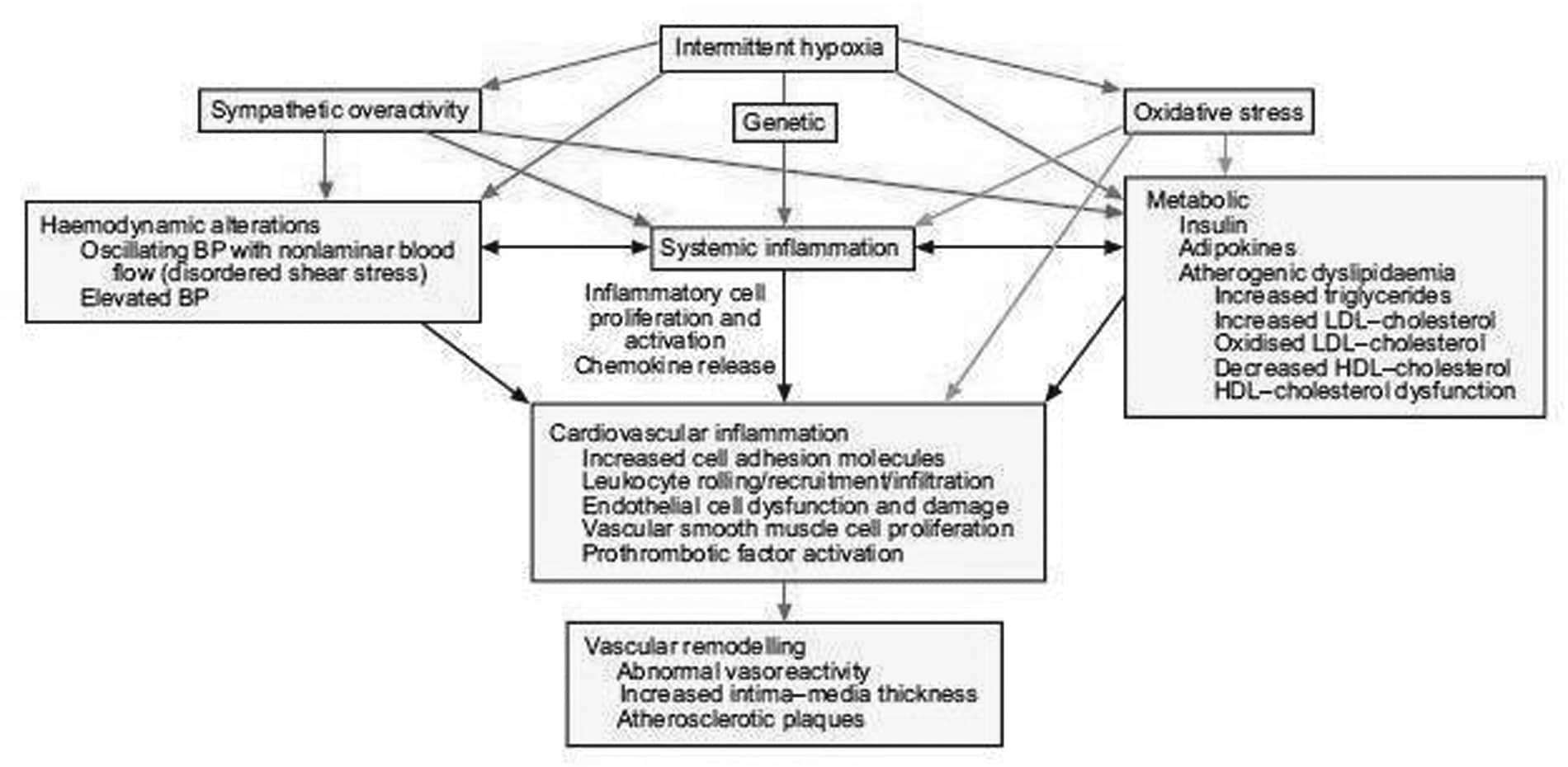
Obstructive sleep apnoea (OSA) syndrome is a highly prevalent sleep disorder leading to cardiovascular and metabolic complications. Intermittent hypoxia (IH) is usually considered as the main trigger for the associated cardiovascular[1] and metabolic alterations. Indeed, the repetitive sequence of hypoxia-reoxygenation due to recurrent pharyngeal collapse during sleep, leads to several consequences such as hemodynamic, hormono-metabolic, oxidative and immuno-inflammatory alterations that may interact and aggravate each other, resulting in artery structural changes, from adaptive to degenerative atherosclerotic remodeling[2, 3]. There are clinical studies as well as studies in the general population supporting a link between OSA and atherosclerosis. Atherosclerosis has been found in OSA patients free of other cardiovascular risk factors, and seems to be related to the severity of nocturnal hypoxia. This has been demonstrated using validated sub-clinical markers e.g. intima media thickness measured by carotid artery echography. Early stages of artery alteration, including functional and structural changes, have been evidenced in both OSA patients[4] and rodents experimentally exposed to IH[5]. Impaired vasoreactivity with endothelial dysfunction and/or increased vasoconstrictive responses due to sympathetic, endothelin and renin-angiotensin systems have been reported, also contributing to vascular remodeling and inflammation both at the systemic and vascular level. Oxidative stress[6], inflammation[7] and vascular remodeling[2] can be directly triggered by IH, further aggravated by the OSA-associated hormono-metabolic alterations, such as insulin resistance, dyslipidemia and adipokine imbalance. We have also recently shown that specific chemokines may be involved in vascular remodeling and that fat tissue (visceral, peri-vascular or intra-organs) may be critical in promoting vascular changes (Arnaud et al, submitted). Finally, as shown in OSA patients and in the animal model, genetic susceptibility, comorbidities including obesity as well high fat diet may aggravate atherosclerosis development or progression. The understanding of the molecular mechanisms may contribute to delineate new targets for prevention strategies and development of new treatment of OSA-related atherosclerosis, especially in patients at risk for cardiovascular disease.
University College Dublin (2009) Proc Physiol Soc 15, SA5
Research Symposium: Sleep Apnoea, intermittent hypoxia and atherosclerosis
P. A. Lévy1,2, C. Arnaud2, M. Dematteis2,1, J. Baguet1, J. Pépin1,2
1. EFCR, Grenoble University, Grenoble, France. 2. Inserm ERI17, Joseph Fourier University, Grenoble, France.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.