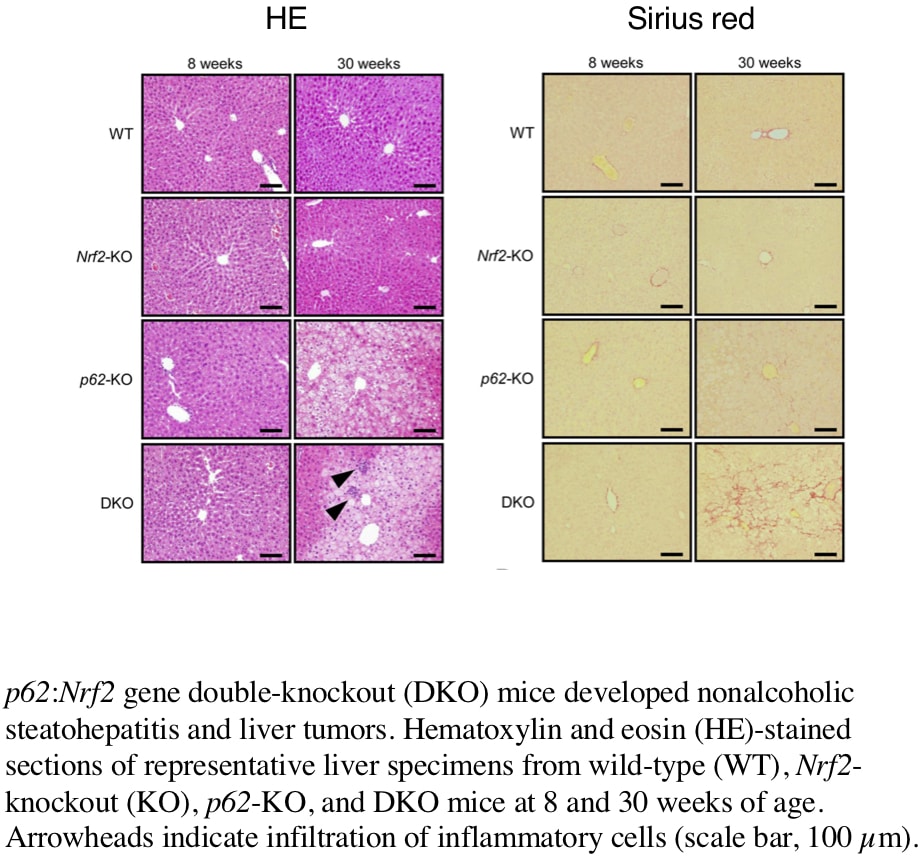
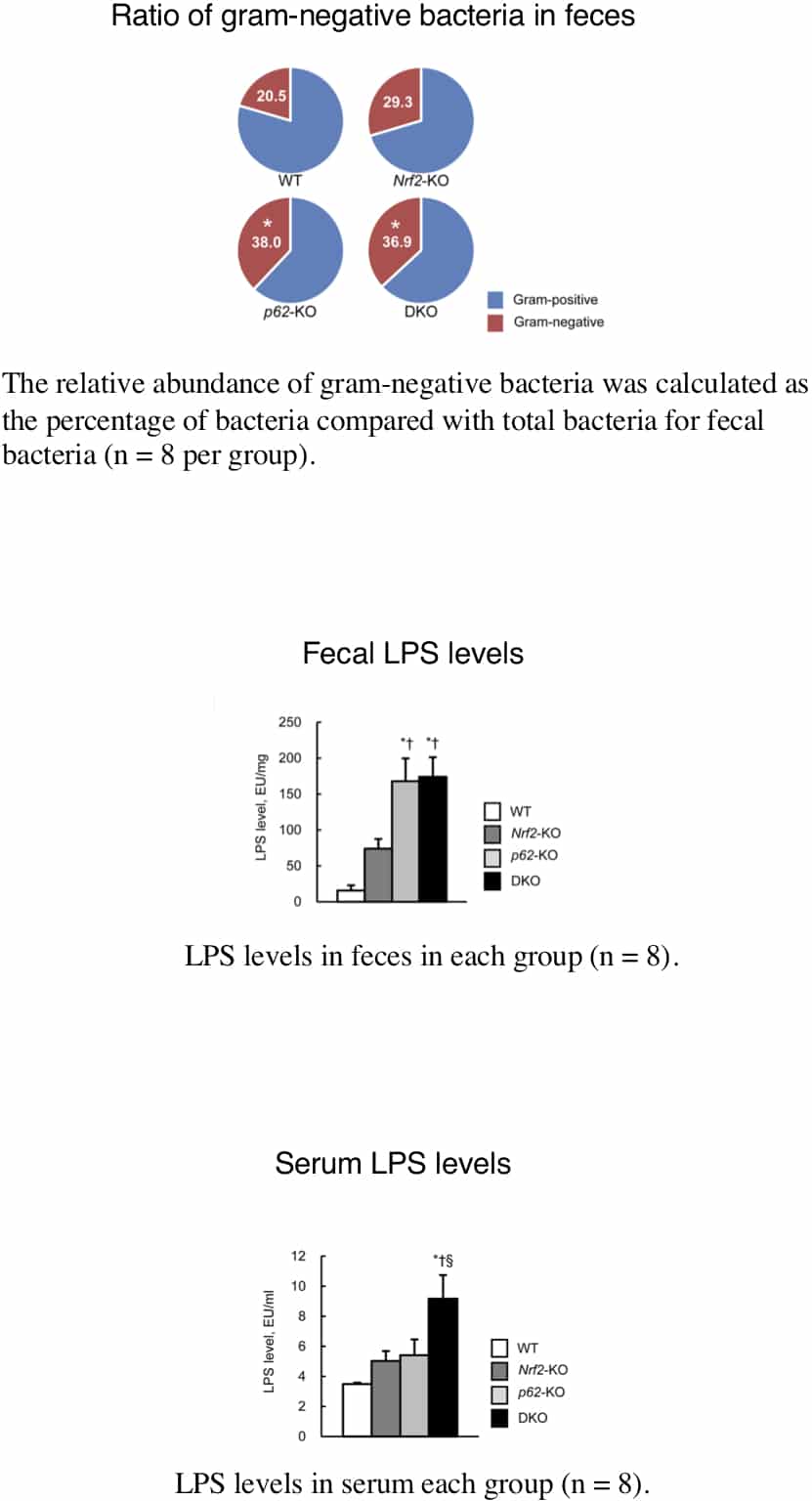
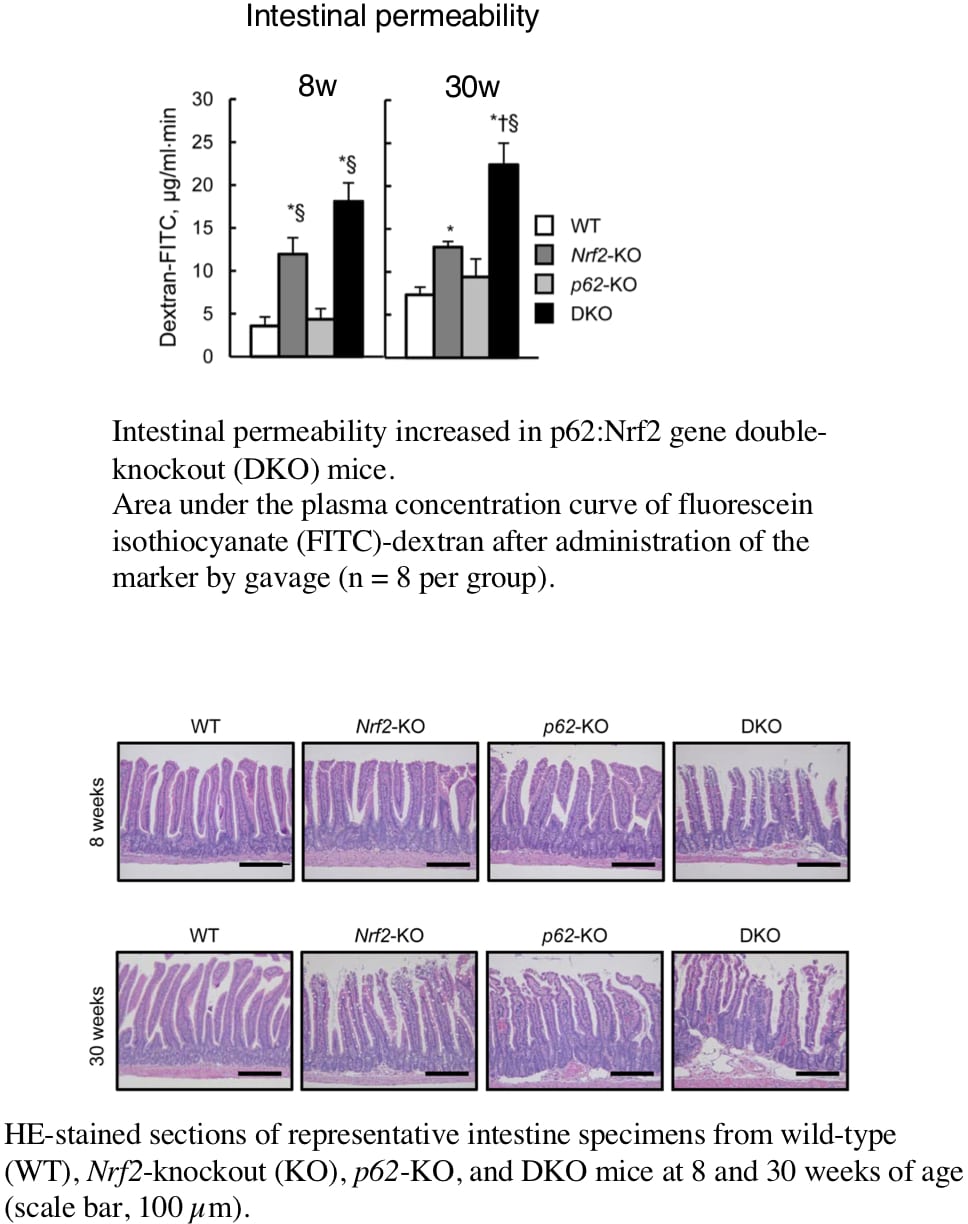
Nonalcoholic steatohepatitis (NASH) is one of the leading causes of chronic liver disease worldwide. However, details of pathogenetic mechanisms remain unknown. In this study, we found deletion of both Sequestosome1(Sqstm1)/p62 and Nrf2 genes spontaneously led to the development of NASH in mice fed a normal chow and was associated with liver tumorigenesis. The pathogenetic mechanisms underlying the NASH development was investigated in p62:Nrf2 double-knockout (DKO) mice. DKO mice showed massive hepatomegaly and steatohepatitis with fat accumulation. DKO mice had hyperphagia[1] and enhanced adipocyte differentiation-induced obesity [2] coupled with insulin resistance and adipokine imbalance. We found that DKO mice also showed dysbiosis associated with an increased proportion of gram-negative bacteria species (wild type:20.5±4.8% vs. DKO:36.9±8.1%, p<0.05, n=8) by fecal 16S rDNA analysis. An increased lipopolysaccharide (LPS) level in the feces was also found in DKO (wild type:15.4±7.7 EU/mg vs. DKO: 176±27 EU/mg, p<0.05, n=8). The intestinal permeability was elevated (wild type:3.8±1.2 dextran-FITC µg/mL·min vs. DKO: 17.3±2.7 dextran-FITC µg/mL·min, p<0.05, n=8) in association with both epithelial damages and decreased expression levels of tight junction protein zona occludens-1 (Zo-1) (relative expression in wild type:1.00±0.08 vs. DKO: 0.67±0.04, p<0.05, n=8), and thereby LPS levels were increased in the serum (wild type:3.1±0.1 EU/mL vs. DKO: 9.0±0.5 EU/mL, p<0.05, n=8) at 8 weeks of age. These results suggest that innate immunity activation by excessive LPS flux from the intestines, occurring both within and outside the liver, is central to the development of hepatic damages in the form of NASH in Sqstm1/p62 and Nrf2 double knockout mice [3].
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, PC160
Poster Communications: Sqstm1/p62 and Nrf2 double knockout mice spontaneously develop nonalcoholic steatohepatitis
E. Warabi1, K. Akiyama1, K. Okada1, T. Yanagawa1, S. Takahashi1, T. Ishii1, J. Shoda1
1. University of Tsukuba, Tsukuba, Japan.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.