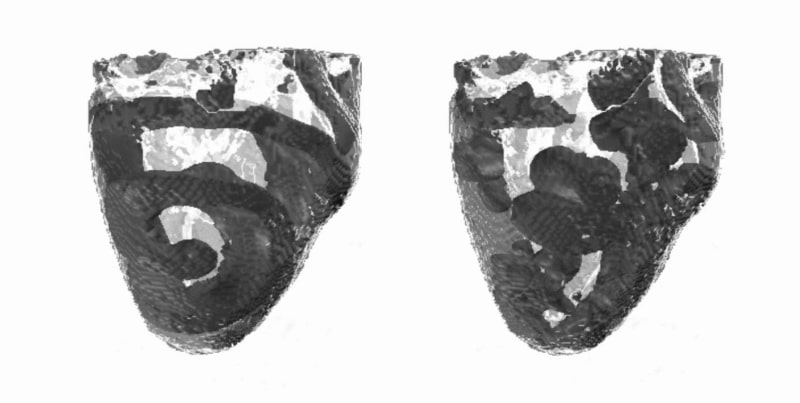
Ventricular fibrillation (VF) is believed to arise by the breakdown of re-entrant scroll waves of excitation. We use a computational model of re-entry and development of VF in a family of homogenous anisotropic canine ventricular architectures that have been algorithmically reconstructed from diffusion tensor magnetic resonance imaging (DT-MRI) datasets (Benson et al. 2005). The model is a monodomain reaction-diffusion system with the Fenton-Karma MBR excitation kinetics (Fenton & Karma, 1998: see Table 1) and anisotropic diffusion within the DT-MRI derived 3D ventricular anatomy. A single re-entrant scroll wave is initiated in the left ventricular free wall (Clayton & Holden, 2004). For the standard parameter set, the scroll breaks down after about 500 ms, evolving into VF. The single filament of the scroll wave (i.e., a phase singularity around which the wave rotates) undergoes a cascade of splittings, which leads to the emergence of multiple irregularly moving filaments. For decreased slow inward Ca2+ current, and hence shorter cellular action potential duration (APD), the scroll is apparently stable (Figure 1), and persists for 3 s. A re-entrant spiral wave initiated in an isotropic 2D tissue is stable for both normal and decreased Ca2+ current. The breakdown of a re-entrant wave in a homogeneous anisotropic tissue can be due to either cellular restitution properties (Garfinkel et al. 2000), or the tissue anisotropy (Fenton & Karma, 1998): our results demonstrate that breakdown due to anisotropy can be prevented by changing excitation parameters that decrease APD and flatten the restitution curve.
University of Bristol (2005) J Physiol 567P, PC10
Poster Communications: Stabilization of re-entry in canine virtual ventricles by decreasing the slow inward Ca2+ current
Aslanidi, Oleg V; Hsu, Edward W; Holden, Arun V;
1. School of Biomedical Sciences & Cardiovascular Research Institute, University of Leeds, Leeds, United Kingdom. 2. Department of Biomedical Engineering, Duke University, Durham, NC, USA.
View other abstracts by:
Figure 1. Re-entry and VF in the DT-MRI virtual ventricles. Snapshots of dark-grey voltage isosurfaces are shown within the pale-grey ventricles at 2 s. The slow inward Ca2+ current is either normal (right) or decreased by 50% (left) whereas the tissue anisotropy is the same in both cases.
Where applicable, experiments conform with Society ethical requirements.