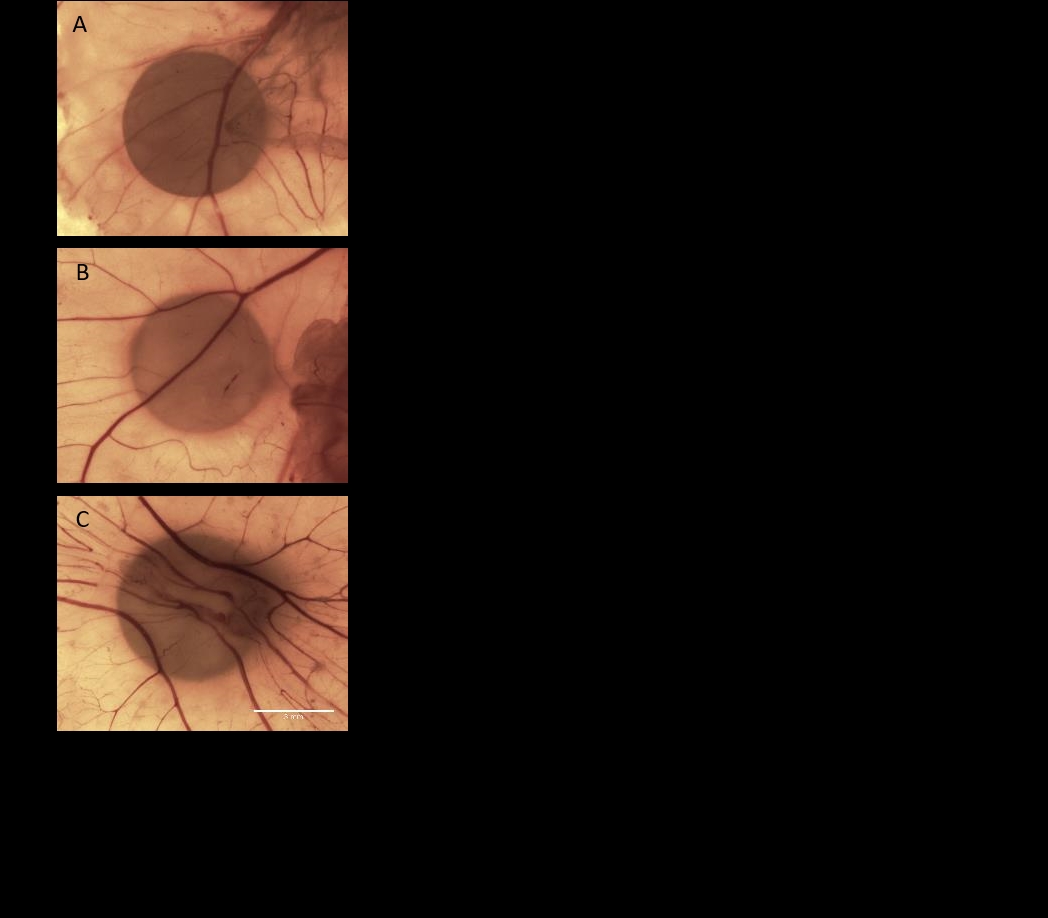
Rationale: Angiogenesis is where new blood vessels form from pre-existing vessels. With adequate oxygen transport and waste removal essential for tissue homeostasis, restrictions in blood supply can lead to ischaemia. Ischaemia due to defective angiogenesis in muscle can contribute to disease pathology in conditions such as atherosclerosis, peripheral artery disease or genetic conditions such as muscular dystrophy. Therapeutic angiogenesis is the co-ordinated delivery of vascular growth factors with the aim of promoting blood vessel growth to re-establish blood flow. Vascular endothelial growth factor (VEGF) is paramount for the induction of angiogenesis, while also playing a role in the promotion of myogenesis, having been shown to stimulate muscle repair following injury, making it an ideal candidate as an angiogenic (and myogenic) stimulant in muscle [1] . The C2C12 immortal cell line consists of isolated murine skeletal muscle cells. Stable transfection, unlike in transient transfection, is where DNA is introduced into a cell line long term, passing the introduced DNA onto their progeny. Hypothesis: VEGF-A expressing C2C12 muscle cells exert a pro-angiogenic effect potentiating therapeutic angiogenesis in muscle disease. Objectives: To establish a muscle cell line stably expressing VEGF-A and to determine its potency in eliciting an angiogenic effect in the chick chorioallantoic membrane (CAM) model of angiogenesis. Methodology: C2C12 cells were transfected with VEGF-GFP and GFP plasmids respectively, and then underwent antibiotic selection to isolate separate high and low expressing cell lines for each transgene. GFP was included to visually inspect for transfection efficiency. ELISA determined VEGF-A concentration secreted from stable cells into cell media and a CAM filter disc assay was used to investigate the angiogenic effect of the C2C12 conditioned media. A CAM Matrigel assay was employed to explore the angiogenic effects the different cell lines directly. Angiogenesis was quantitated using a standard stereological scoring method applied to images of the CAMs [2] . Findings: Herein, we successfully have characterised VEGF-A expression and the angiogenic potential of stably transfected C2C12 mouse myoblast cells using the CAM assay. Values are expressed as means ± S.E.M., compared by ANOVA. VEGF-A stable transfected C2C12 cells secreted significantly increased amount of VEGF-A, compared to GFP transfected alone (VEGF-A: 10198 ± 230.3 pg/mL vs GFP: 1081 ± 30.9 pg/mL, p<0.01). Conditioned media from cells produced a significant increase in angiogenesis in the CAM filter disc assay (Non-transfected: 15.4 ± 1.5; GFP: 15.5 ± 1.8; VEGF: 23.9 ± 2.1; p<0.01) (fig. 1). The CAM Matrigel assay determined that stable transfected C2C12 cells secreted VEGF-A which produced an angiogenic response when applied directly to the CAM assay (GFP: 6.3 ± 2.1 vs VEGF: 15.0 ± 1.2; p<0.01). In summary, C2C12 cells were successfully stably transfected to secrete elevated levels of VEGF-A and are proven to elicit a pro-angiogenic effect in the CAM model of angiogenesis. These studies qualify the potential use of a genetically modified muscle cell line in therapeutic angiogenesis for the treatment of muscle disease associated with vascular defects.
Future Physiology 2021 (Virutal) (2021) Proc Physiol Soc 47, PC06
Poster Communications: Stable Expression of VEGF-A in C2C12 Muscle Cells and Angiogenic Potential
Donna C. Kennedy1, Antony M. Wheatley1, Karl J.A. McCullagh1
1 Department of Physiology, School of Medicine, Human Biology Building, National University of Ireland Galway, Galway, Ireland
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.